Genetic Mouse Models with Intestinal-Specific Tight Junction Deletion Resemble an Ulcerative Colitis Phenotype
- PMID: 28575164
- PMCID: PMC5881657
- DOI: 10.1093/ecco-jcc/jjx075
Genetic Mouse Models with Intestinal-Specific Tight Junction Deletion Resemble an Ulcerative Colitis Phenotype
Abstract
Background and aims: A key pathogenetic feature of ulcerative colitis [UC] is an intrinsic low mucus phosphatidylcholine[PC] content. Recently, a paracellular transport for PC across tight junctions[TJs] was described, suggesting TJ disturbance as a cause of diminished luminal PC transport. Therefore, we aimed to generate mutant mice with TJ deletion to evaluate whether a UC phenotype developed.
Methods: CL57BL/6 control wild-type mice were compared to mutant mice with tamoxifen-induced villin-Cre-dependent intestinal deletion of kindlin 1 and 2.
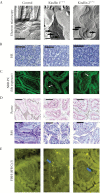
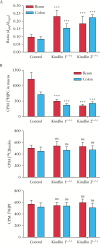
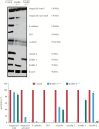
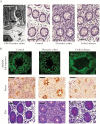
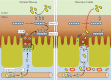
Results: Electron microscopy of mucosal biopsies obtained from both mutants before overt inflammation following only 2 days of tamoxifen exposure revealed a defective TJ morphology with extended paracellular space and, by light microscopy, expanded mucosal crypt lumina. PC secretion into mucus was reduced by >65% and the mucus PC content dropped by >50%, causing a >50 % decrease of mucus hydrophobicity in both mutants. Consequently, the microbiota was able to penetrate the submucosa. After 3 days of tamoxifen exposure, intestinal inflammation was present in both mutants, with loose bloody stools as well as macroscopic and histological features of colitis. Oral PC supplementation was able to suppress inflammation. By analogy, colonic biopsies obtained from patients with UC in remission also showed a defective epithelium with widened intercellular clefts, and enlarged crypt luminal diameters with functionally impaired luminal PC secretion.
Conclusions: Genetic mouse models with intestinal deletion of kindlin 1 and 2 resulted in TJ deletion and revealed pathophysiological features of impaired PC secretion to the mucus leading to mucosal inflammation compatible with human UC.
Keywords: Mucosal barrier; hydrophobicity; mucus; phosphatidylcholine; ulcerative colitis.
Copyright © 2017 European Crohn’s and Colitis Organisation (ECCO). Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com
Figures

Similar articles
-
The neglected biliary mucus and its phosphatidylcholine content: a putative player in pathogenesis of primary cholangitis-a narrative review article.Ann Transl Med. 2021 Apr;9(8):738. doi: 10.21037/atm-20-3591. Ann Transl Med. 2021. PMID: 33987436 Free PMC article. Review.
-
Phosphatidylcholine passes through lateral tight junctions for paracellular transport to the apical side of the polarized intestinal tumor cell-line CaCo2.Biochim Biophys Acta. 2016 Sep;1861(9 Pt A):1161-1169. doi: 10.1016/j.bbalip.2016.06.019. Epub 2016 Jun 27. Biochim Biophys Acta. 2016. PMID: 27365309
-
Spontaneous colitis in Muc2-deficient mice reflects clinical and cellular features of active ulcerative colitis.PLoS One. 2014 Jun 19;9(6):e100217. doi: 10.1371/journal.pone.0100217. eCollection 2014. PLoS One. 2014. PMID: 24945909 Free PMC article.
-
Interdependence between Chromogranin-A, Alternatively Activated Macrophages, Tight Junction Proteins and the Epithelial Functions. A Human and In-Vivo/In-Vitro Descriptive Study.Int J Mol Sci. 2020 Oct 27;21(21):7976. doi: 10.3390/ijms21217976. Int J Mol Sci. 2020. PMID: 33121008 Free PMC article.
-
[Mucosal protection by phosphatidylcholine as new therapeutic concept in ulcerative colitis].Z Gastroenterol. 2013 Apr;51(4):384-9. doi: 10.1055/s-0033-1335042. Epub 2013 Apr 12. Z Gastroenterol. 2013. PMID: 23585269 Review. German.
Cited by
-
Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies.Front Immunol. 2020 Sep 4;11:2054. doi: 10.3389/fimmu.2020.02054. eCollection 2020. Front Immunol. 2020. PMID: 33013869 Free PMC article. Review.
-
Sini San ameliorates duodenal mucosal barrier injury and low‑grade inflammation via the CRF pathway in a rat model of functional dyspepsia.Int J Mol Med. 2020 Jan;45(1):53-60. doi: 10.3892/ijmm.2019.4394. Epub 2019 Nov 4. Int J Mol Med. 2020. PMID: 31746413 Free PMC article.
-
Milk Exosomes Prevent Intestinal Inflammation in a Genetic Mouse Model of Ulcerative Colitis: A Pilot Experiment.Inflamm Intest Dis. 2020 Aug;5(3):117-123. doi: 10.1159/000507626. Epub 2020 May 20. Inflamm Intest Dis. 2020. PMID: 32999884 Free PMC article.
-
The mechanism of traditional medicine in alleviating ulcerative colitis: regulating intestinal barrier function.Front Pharmacol. 2023 Oct 9;14:1228969. doi: 10.3389/fphar.2023.1228969. eCollection 2023. Front Pharmacol. 2023. PMID: 37876728 Free PMC article. Review.
-
The Detergent Effect of Mesalazine Interferes with Phosphatidylcholine Binding to Mucin 2.Inflamm Intest Dis. 2019 Feb;3(3):107-115. doi: 10.1159/000493347. Epub 2018 Oct 18. Inflamm Intest Dis. 2019. PMID: 30820432 Free PMC article.
References
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–29. - PubMed
-
- Kao YC, Lichtenberger LM. Phospholipid- and neutral lipid-containing organelles of rat gastroduodenal mucous cells. Possible origin of the hydrophobic mucosal lining. Gastroenterology 1991;101:7–21. - PubMed
-
- Ehehalt R, Jochims C, Lehmann WD, et al. Evidence of luminal phosphatidylcholine secretion in rat ileum. Biochim Biophys Acta 2004;1682:63–71. - PubMed
-
- Stremmel W, Ehehalt R, Staffer S, et al. Mucosal protection by phosphatidylcholine. Dig Dis 2012;30(Suppl 3):85–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

