Development of plant-produced protein body vaccine candidates for bluetongue virus
- PMID: 28558675
- PMCID: PMC5450216
- DOI: 10.1186/s12896-017-0370-5
Development of plant-produced protein body vaccine candidates for bluetongue virus
Abstract
Background: Bluetongue is a disease of domestic and wild ruminants caused by bluetongue virus serotypes (BTV), which have caused serious outbreaks worldwide. Commercially available vaccines are live-attenuated or inactivated virus strains: these are effective, but there is the risk of reversion to virulence or reassortment with circulating strains for live virus, and residual live virus for the inactivated vaccines. The live-attenuated virus vaccines are not able to distinguish naturally infected animals from vaccinated animals (DIVA compliant). Recombinant vaccines are preferable to minimize the risks associated with these vaccines, and would also enable the development of candidate vaccines that are DIVA-compliant.
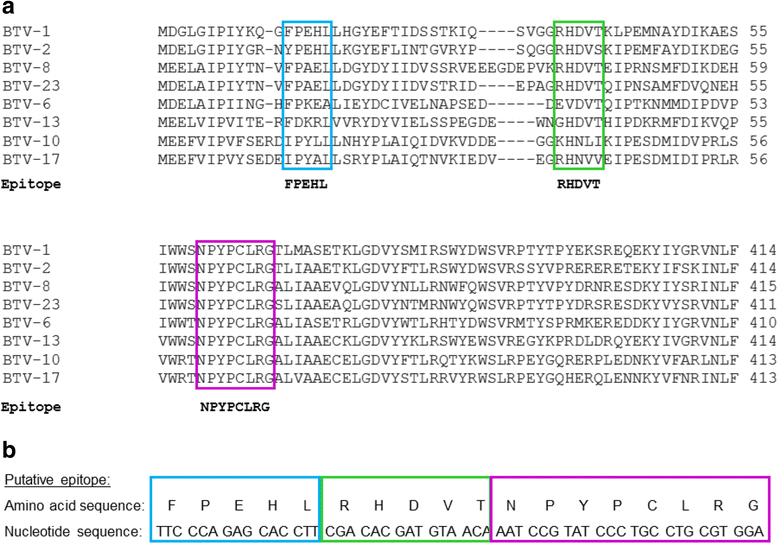
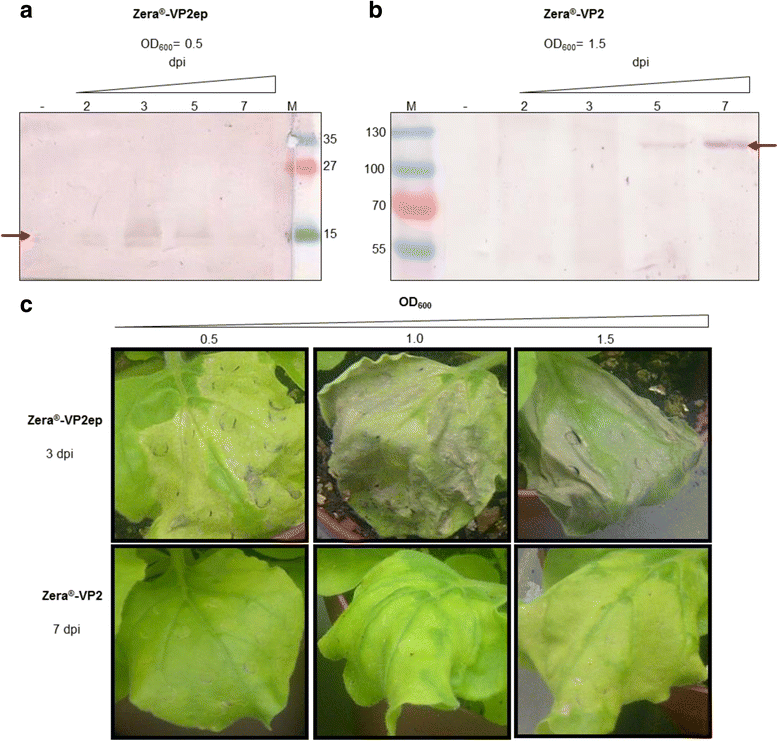
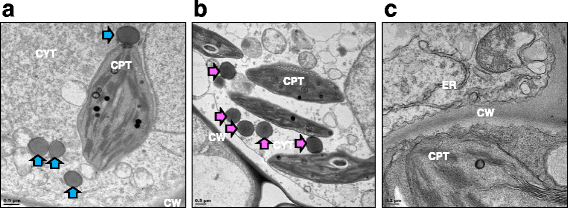
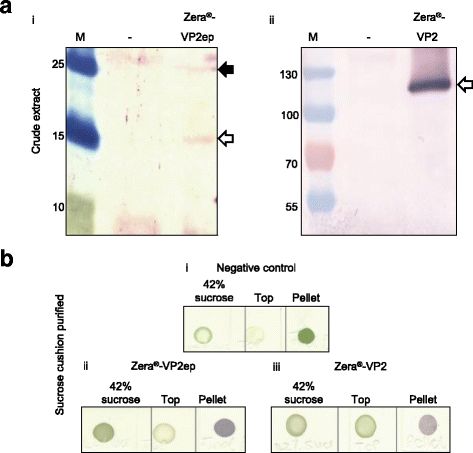
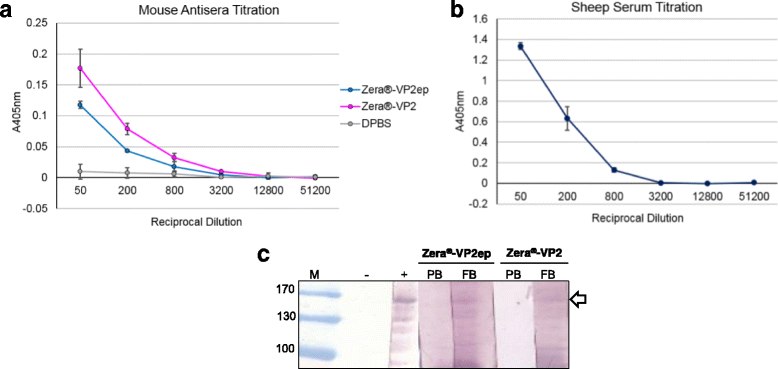
Results: In this study, two novel protein body (PB) plant-produced vaccines were developed, Zera®-VP2ep and Zera®-VP2. Zera®-VP2ep contained B-cell epitope sequences of multiple BTV serotypes and Zera®-VP2 contained the full-length BTV-8 VP2 codon-optimised sequence. In addition to fulfilling the DIVA requirement, Zera®-VP2ep was aimed at being multivalent with the ability to stimulate an immune response to several BTV serotypes. Both these candidate vaccines were successfully made in N. benthamiana via transient Agrobacterium-mediated expression, and in situ TEM analysis showed that the expressed proteins accumulated within the cytoplasm of plant cells in dense membrane-defined PBs. The peptide sequences included in Zera®-VP2ep contained epitopes that bound antibodies produced against native VP2. Preliminary murine immunogenicity studies showed that the PB vaccine candidates elicited anti-VP2 immune responses in mice without the use of adjuvant.
Conclusions: These proof of concept results demonstrate that Zera®-VP2ep and Zera®-VP2 have potential as BTV vaccines and their development should be further investigated.
Keywords: Bluetongue virus; Nicotiana benthamiana; Protein body; Vaccine; Zera®.
Figures
Similar articles
-
VP2-serotyped live-attenuated bluetongue virus without NS3/NS3a expression provides serotype-specific protection and enables DIVA.Vaccine. 2014 Dec 12;32(52):7108-14. doi: 10.1016/j.vaccine.2014.10.033. Epub 2014 Oct 31. Vaccine. 2014. PMID: 25454873
-
Application of bluetongue Disabled Infectious Single Animal (DISA) vaccine for different serotypes by VP2 exchange or incorporation of chimeric VP2.Vaccine. 2015 Feb 4;33(6):812-8. doi: 10.1016/j.vaccine.2014.12.003. Epub 2014 Dec 13. Vaccine. 2015. PMID: 25510389
-
Plant-produced Bluetongue chimaeric VLP vaccine candidates elicit serotype-specific immunity in sheep.Vaccine. 2019 Sep 24;37(41):6068-6075. doi: 10.1016/j.vaccine.2019.08.042. Epub 2019 Aug 27. Vaccine. 2019. PMID: 31471154
-
Recombinant vaccines against bluetongue virus.Virus Res. 2014 Mar;182:78-86. doi: 10.1016/j.virusres.2013.11.013. Epub 2013 Nov 25. Virus Res. 2014. PMID: 24287057 Review.
-
Genetically engineered multi-component virus-like particles as veterinary vaccines.Immunol Cell Biol. 1993 Oct;71 ( Pt 5):381-9. doi: 10.1038/icb.1993.44. Immunol Cell Biol. 1993. PMID: 8270267 Review.
Cited by
-
Plant-derived protein bodies as delivery vehicles for recombinant proteins into mammalian cells.Biotechnol Bioeng. 2020 Apr;117(4):1037-1047. doi: 10.1002/bit.27273. Epub 2020 Jan 30. Biotechnol Bioeng. 2020. PMID: 31956981 Free PMC article.
-
A protective bivalent vaccine against Rift Valley fever and bluetongue.NPJ Vaccines. 2020 Jul 30;5(1):70. doi: 10.1038/s41541-020-00218-y. eCollection 2020. NPJ Vaccines. 2020. PMID: 32793399 Free PMC article.
-
Evaluation of the immunogenicity of a Crimean-Congo hemorrhagic fever virus vaccine candidate in mice developed based on a baculovirus Zera nanoparticle delivery system.Front Vet Sci. 2023 Jun 1;10:1126785. doi: 10.3389/fvets.2023.1126785. eCollection 2023. Front Vet Sci. 2023. PMID: 37323845 Free PMC article.
-
Characterization and Immunogenicity of HIV Envelope gp140 Zera® Tagged Antigens.Front Bioeng Biotechnol. 2020 Apr 9;8:321. doi: 10.3389/fbioe.2020.00321. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32328488 Free PMC article.
-
Microparticles and Nanoparticles from Plants-The Benefits of Bioencapsulation.Vaccines (Basel). 2021 Apr 11;9(4):369. doi: 10.3390/vaccines9040369. Vaccines (Basel). 2021. PMID: 33920425 Free PMC article. Review.
References
-
- Vellema P. Bluetongue in sheep: question marks on bluetongue virus serotype 8 in Europe. Small Rumin Res. 2008;76(1–2):141–8. doi: 10.1016/j.smallrumres.2007.12.009. - DOI
-
- du Toit RM. The transmission of bluetongue and horse sickness by Culicoides. Onderstepoort J Vet Sci Anim Ind. 1944;7:16–390.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

