Factors Released from Endothelial Cells Exposed to Flow Impact Adhesion, Proliferation, and Fate Choice in the Adult Neural Stem Cell Lineage
- PMID: 28557666
- PMCID: PMC5564022
- DOI: 10.1089/scd.2016.0350
Factors Released from Endothelial Cells Exposed to Flow Impact Adhesion, Proliferation, and Fate Choice in the Adult Neural Stem Cell Lineage
Abstract
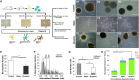
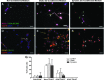
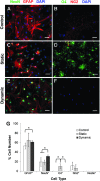
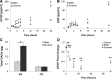
The microvasculature within the neural stem cell (NSC) niche promotes self-renewal and regulates lineage progression. Previous work identified endothelial-produced soluble factors as key regulators of neural progenitor cell (NPC) fate and proliferation; however, endothelial cells (ECs) are sensitive to local hemodynamics, and the effect of this key physiological process has not been defined. In this study, we evaluated adult mouse NPC response to soluble factors isolated from static or dynamic (flow) EC cultures. Endothelial factors generated under dynamic conditions significantly increased neuronal differentiation, while those released under static conditions stimulated oligodendrocyte differentiation. Flow increases EC release of neurogenic factors and of heparin sulfate glycosaminoglycans that increase their bioactivity, likely underlying the enhanced neuronal differentiation. Additionally, endothelial factors, especially from static conditions, promoted adherent growth. Together, our data suggest that blood flow may impact proliferation, adhesion, and the neuron-glial fate choice of adult NPCs, with implications for diseases and aging that reduce flow.
Keywords: neural stem cells; neurogenesis; oligodendrocytes; shear stress; vascular niche.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage.J Neurosci. 2015 Mar 18;35(11):4528-39. doi: 10.1523/JNEUROSCI.1188-14.2015. J Neurosci. 2015. PMID: 25788671 Free PMC article.
-
Protein S Regulates Neural Stem Cell Quiescence and Neurogenesis.Stem Cells. 2017 Mar;35(3):679-693. doi: 10.1002/stem.2522. Epub 2016 Nov 8. Stem Cells. 2017. PMID: 27753164
-
Continuous live imaging of adult neural stem cell division and lineage progression in vitro.Development. 2011 Mar;138(6):1057-68. doi: 10.1242/dev.061663. Development. 2011. PMID: 21343361
-
From the vascular microenvironment to neurogenesis.Brain Res Bull. 2011 Jan 15;84(1):1-7. doi: 10.1016/j.brainresbull.2010.09.008. Epub 2010 Sep 17. Brain Res Bull. 2011. PMID: 20850508 Review.
-
Glial control of neurogenesis.Curr Opin Neurobiol. 2017 Dec;47:188-195. doi: 10.1016/j.conb.2017.10.025. Epub 2017 Nov 13. Curr Opin Neurobiol. 2017. PMID: 29145015 Review.
Cited by
-
New Insight Into Neutrophils: A Potential Therapeutic Target for Cerebral Ischemia.Front Immunol. 2021 Jul 14;12:692061. doi: 10.3389/fimmu.2021.692061. eCollection 2021. Front Immunol. 2021. PMID: 34335600 Free PMC article. Review.
-
NSCs Under Strain-Unraveling the Mechanoprotective Role of Differentiating Astrocytes in a Cyclically Stretched Coculture With Differentiating Neurons.Front Cell Neurosci. 2021 Sep 24;15:706585. doi: 10.3389/fncel.2021.706585. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34630042 Free PMC article.
-
Endothelial Cells Exposed to Fluid Shear Stress Support Diffusion Based Maturation of Adult Neural Progenitor Cells.Cell Mol Bioeng. 2017 Dec 1;11(2):117-130. doi: 10.1007/s12195-017-0516-5. eCollection 2018 Apr. Cell Mol Bioeng. 2017. PMID: 31719881 Free PMC article.
-
Bioengineering the neurovascular niche to study the interaction of neural stem cells and endothelial cells.APL Bioeng. 2021 Mar 3;5(1):011507. doi: 10.1063/5.0027211. eCollection 2021 Mar. APL Bioeng. 2021. PMID: 33688617 Free PMC article. Review.
References
-
- Louissaint A, Jr., Rao S, Leventhal C. and Goldman SA. (2002). Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34:945–960 - PubMed
-
- Reynolds BA. and Weiss S. (1992). Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710 - PubMed
-
- Palmer TD, Willhoite AR. and Gage FH. (2000). Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425:479–494 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

