Pathways to zoonotic spillover
- PMID: 28555073
- PMCID: PMC5791534
- DOI: 10.1038/nrmicro.2017.45
Pathways to zoonotic spillover
Abstract
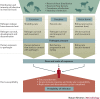
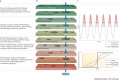
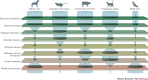
Zoonotic spillover, which is the transmission of a pathogen from a vertebrate animal to a human, presents a global public health burden but is a poorly understood phenomenon. Zoonotic spillover requires several factors to align, including the ecological, epidemiological and behavioural determinants of pathogen exposure, and the within-human factors that affect susceptibility to infection. In this Opinion article, we propose a synthetic framework for animal-to-human transmission that integrates the relevant mechanisms. This framework reveals that all zoonotic pathogens must overcome a hierarchical series of barriers to cause spillover infections in humans. Understanding how these barriers are functionally and quantitatively linked, and how they interact in space and time, will substantially improve our ability to predict or prevent spillover events. This work provides a foundation for transdisciplinary investigation of spillover and synthetic theory on zoonotic transmission.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Infection and disease in reservoir and spillover hosts: determinants of pathogen emergence.Curr Top Microbiol Immunol. 2007;315:113-31. doi: 10.1007/978-3-540-70962-6_6. Curr Top Microbiol Immunol. 2007. PMID: 17848063 Review.
-
Ecological interventions to prevent and manage zoonotic pathogen spillover.Philos Trans R Soc Lond B Biol Sci. 2019 Sep 30;374(1782):20180342. doi: 10.1098/rstb.2018.0342. Epub 2019 Aug 12. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31401951 Free PMC article. Review.
-
Spillover and pandemic properties of zoonotic viruses with high host plasticity.Sci Rep. 2015 Oct 7;5:14830. doi: 10.1038/srep14830. Sci Rep. 2015. PMID: 26445169 Free PMC article.
-
A quantitative prioritisation of human and domestic animal pathogens in Europe.PLoS One. 2014 Aug 19;9(8):e103529. doi: 10.1371/journal.pone.0103529. eCollection 2014. PLoS One. 2014. PMID: 25136810 Free PMC article.
-
Sustained fecal-oral human-to-human transmission following a zoonotic event.Curr Opin Virol. 2017 Feb;22:1-6. doi: 10.1016/j.coviro.2016.11.001. Epub 2016 Nov 23. Curr Opin Virol. 2017. PMID: 27888698 Free PMC article. Review.
Cited by
-
Human outbreaks of a novel reassortant Oropouche virus in the Brazilian Amazon region.Nat Med. 2024 Dec;30(12):3509-3521. doi: 10.1038/s41591-024-03300-3. Epub 2024 Sep 18. Nat Med. 2024. PMID: 39293488
-
Ecological drivers of dog heartworm transmission in California.Parasit Vectors. 2022 Oct 23;15(1):388. doi: 10.1186/s13071-022-05526-x. Parasit Vectors. 2022. PMID: 36274157 Free PMC article.
-
Species misidentification in local markets: Discrepancies between reporting and molecular identification of bushmeat species in northern Uganda.One Health. 2021 Apr 19;13:100251. doi: 10.1016/j.onehlt.2021.100251. eCollection 2021 Dec. One Health. 2021. PMID: 33997235 Free PMC article.
-
Incorporating environmental heterogeneity and observation effort to predict host distribution and viral spillover from a bat reservoir.Proc Biol Sci. 2023 Nov 29;290(2011):20231739. doi: 10.1098/rspb.2023.1739. Epub 2023 Nov 22. Proc Biol Sci. 2023. PMID: 37989240 Free PMC article.
-
Corrigendum: Evolution, ecology, and zoonotic transmission of betacoronaviruses: A review.Front Vet Sci. 2023 Feb 8;10:1147940. doi: 10.3389/fvets.2023.1147940. eCollection 2023. Front Vet Sci. 2023. PMID: 36846266 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

