Inhibition of Lysyl Oxidases Impairs Migration and Angiogenic Properties of Tumor-Associated Pericytes
- PMID: 28553358
- PMCID: PMC5434472
- DOI: 10.1155/2017/4972078
Inhibition of Lysyl Oxidases Impairs Migration and Angiogenic Properties of Tumor-Associated Pericytes
Abstract
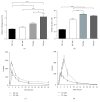
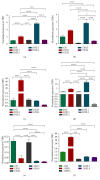
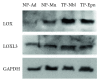
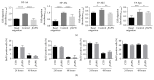
Pericytes are important cellular components of the tumor microenviroment with established roles in angiogenesis and metastasis. These two cancer hallmarks are modulated by enzymes of the LOX family, but thus far, information about LOX relevance in tumor-associated pericytes is lacking. Here, we performed a comparative characterization of normal and tumoral pericytes and report for the first time the modulatory effects of LOX enzymes on activated pericyte properties. Tumoral pericytes isolated from childhood ependymoma and neuroblastoma specimens displayed angiogenic properties in vitro and expressed typical markers, including CD146, NG2, and PDGFRβ. Expression of all LOX family members could be detected in both normal and tumor-associated pericytes. In most pericyte samples, LOXL3 was the family member displaying the highest transcript levels. Inhibition of LOX/LOXL activity with the inhibitor β-aminopropionitrile (βAPN) significantly reduced migration of pericytes, while proliferation rates were kept unaltered. Formation of tube-like structures in vitro by pericytes was also significantly impaired upon inhibition of LOX/LOXL activity with βAPN, which induced more prominent effects in tumor-associated pericytes. These findings reveal a novel involvement of the LOX family of enzymes in migration and angiogenic properties of pericytes, with implications in tumor development and in therapeutic targeting tumor microenvironment constituents.
Figures

Similar articles
-
Purification of enzymatically active human lysyl oxidase and lysyl oxidase-like protein from Escherichia coli inclusion bodies.Protein Expr Purif. 2003 Oct;31(2):240-6. doi: 10.1016/s1046-5928(03)00217-1. Protein Expr Purif. 2003. PMID: 14550642
-
The NG2 Proteoglycan in Pericyte Biology.Adv Exp Med Biol. 2018;1109:5-19. doi: 10.1007/978-3-030-02601-1_2. Adv Exp Med Biol. 2018. PMID: 30523586 Review.
-
Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism.Cancer Res. 2005 Dec 15;65(24):11429-36. doi: 10.1158/0008-5472.CAN-05-1274. Cancer Res. 2005. PMID: 16357151
-
Lysyl oxidase and the lysyl oxidase-like protein modulate odontoblastic differentiation of human dental pulp cells.J Endod. 2012 Jun;38(6):769-73. doi: 10.1016/j.joen.2012.03.014. Epub 2012 Apr 21. J Endod. 2012. PMID: 22595110
-
Lysyl oxidases: a novel multifunctional amine oxidase family.Prog Nucleic Acid Res Mol Biol. 2001;70:1-32. doi: 10.1016/s0079-6603(01)70012-8. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11642359 Review.
Cited by
-
Mutual concessions and compromises between stromal cells and cancer cells: driving tumor development and drug resistance.Cell Oncol (Dordr). 2018 Aug;41(4):353-367. doi: 10.1007/s13402-018-0388-2. Epub 2018 Jul 19. Cell Oncol (Dordr). 2018. PMID: 30027403 Review.
-
Targeting pericytes for neurovascular regeneration.Cell Commun Signal. 2019 Mar 20;17(1):26. doi: 10.1186/s12964-019-0340-8. Cell Commun Signal. 2019. PMID: 30894190 Free PMC article. Review.
-
Recent Advances in Liver Cancer Stem Cells: Non-coding RNAs, Oncogenes and Oncoproteins.Front Cell Dev Biol. 2020 Oct 7;8:548335. doi: 10.3389/fcell.2020.548335. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33117795 Free PMC article. Review.
-
LOXL3 Silencing Hampers the Metastasis and Angiogenesis of Gastric Cancer Cells Dependent on Ferroptosis Activation.Mol Biotechnol. 2024 Aug 27. doi: 10.1007/s12033-024-01229-z. Online ahead of print. Mol Biotechnol. 2024. PMID: 39192165
-
Cellular and Molecular Heterogeneity Associated with Vessel Formation Processes.Biomed Res Int. 2018 Oct 10;2018:6740408. doi: 10.1155/2018/6740408. eCollection 2018. Biomed Res Int. 2018. PMID: 30406137 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

