Chronic hypoxia attenuates the vasodilator efficacy of protein kinase G in fetal and adult ovine cerebral arteries
- PMID: 28550175
- PMCID: PMC5538865
- DOI: 10.1152/ajpheart.00480.2016
Chronic hypoxia attenuates the vasodilator efficacy of protein kinase G in fetal and adult ovine cerebral arteries
Abstract
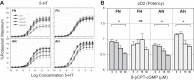
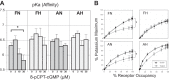
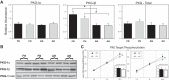
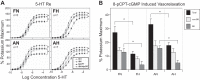
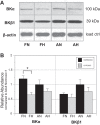
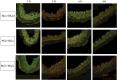
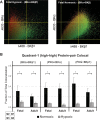
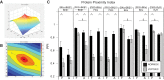
Long-term hypoxia (LTH) attenuates nitric oxide-induced vasorelaxation in ovine middle cerebral arteries. Because cGMP-dependent protein kinase (PKG) is an important mediator of NO signaling in vascular smooth muscle, we tested the hypothesis that LTH diminishes the ability of PKG to interact with target proteins and cause vasorelaxation. Prominent among proteins that regulate vascular tone is the large-conductance Ca2+-sensitive K+ (BK) channel, which is a substrate for PKG and is responsive to phosphorylation on multiple serine/threonine residues. Given the influence of these proteins, we also examined whether LTH attenuates PKG and BK channel protein abundances and PKG activity. Middle cerebral arteries were harvested from normoxic and hypoxic (altitude of 3,820 m for 110 days) fetal and adult sheep. These arteries were denuded and equilibrated with 95% O2-5% CO2 in the presence of N-nitro-l-arginine methyl ester (l-NAME) to inhibit potential confounding influences of events upstream from PKG. Expression and activity of PKG-I were not significantly affected by chronic hypoxia in either fetal or adult arteries. Pretreatment with the BK inhibitor iberiotoxin attenuated vasorelaxation induced by 8-(4-chlorophenylthio)guanosine 3',5'-cyclic monophosphate in normoxic but not LTH arteries. The spatial proximities of PKG with BK channel α- and β1-proteins were examined using confocal microscopy, which revealed a strong dissociation of PKG with these proteins after LTH. These results support our hypothesis that hypoxia reduces the ability of PKG to attenuate vasoconstriction in part through suppression of the ability of PKG to associate with and thereby activate BK channels in arterial smooth muscle.NEW & NOTEWORTHY Using measurements of contractility, protein abundance, kinase activity, and confocal colocalization in fetal and adult ovine cerebral arteries, the present study demonstrates that long-term hypoxia diminishes the ability of cGMP-dependent protein kinase (PKG) to cause vasorelaxation through suppression of its colocalization and interaction with large-conductance Ca2+-sensitive K+ (BK) channel proteins in cerebrovascular smooth muscle. These experiments are among the first to demonstrate hypoxic changes in BK subunit abundances in fetal cerebral arteries and also introduce the use of advanced methods of confocal colocalization to study interaction between PKG and its targets.
Keywords: cGMP; chronic hypoxia; colocalization; confocal; guanylate cyclase; iberiotoxin.
Copyright © 2017 the American Physiological Society.
Figures
Similar articles
-
Hypoxic depression of PKG-mediated inhibition of serotonergic contraction in ovine carotid arteries.Am J Physiol Regul Integr Comp Physiol. 2013 May 1;304(9):R734-43. doi: 10.1152/ajpregu.00212.2012. Epub 2013 Feb 27. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23447135 Free PMC article.
-
Long-term hypoxia increases calcium affinity of BK channels in ovine fetal and adult cerebral artery smooth muscle.Am J Physiol Heart Circ Physiol. 2015 Apr 1;308(7):H707-22. doi: 10.1152/ajpheart.00564.2014. Epub 2015 Jan 16. Am J Physiol Heart Circ Physiol. 2015. PMID: 25599571 Free PMC article.
-
Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase.Am J Physiol Heart Circ Physiol. 2012 Jan 1;302(1):H115-23. doi: 10.1152/ajpheart.00046.2011. Epub 2011 Nov 11. Am J Physiol Heart Circ Physiol. 2012. PMID: 22081702 Free PMC article.
-
Redox regulation of guanylate cyclase and protein kinase G in vascular responses to hypoxia.Respir Physiol Neurobiol. 2010 Dec 31;174(3):259-64. doi: 10.1016/j.resp.2010.08.024. Epub 2010 Sep 8. Respir Physiol Neurobiol. 2010. PMID: 20831906 Free PMC article. Review.
-
Fetal cerebrovascular acclimatization responses to high-altitude, long-term hypoxia: a model for prenatal programming of adult disease?Am J Physiol Regul Integr Comp Physiol. 2005 Jan;288(1):R16-24. doi: 10.1152/ajpregu.00462.2004. Am J Physiol Regul Integr Comp Physiol. 2005. PMID: 15590993 Review.
Cited by
-
Hypoxic Regulation of the Large-Conductance, Calcium and Voltage-Activated Potassium Channel, BK.Front Physiol. 2021 Dec 22;12:780206. doi: 10.3389/fphys.2021.780206. eCollection 2021. Front Physiol. 2021. PMID: 35002762 Free PMC article. Review.
-
Prenatal metyrapone treatment modulates neonatal cerebrovascular structure, function, and vulnerability to mild hypoxic-ischemic injury.Am J Physiol Regul Integr Comp Physiol. 2020 Jan 1;318(1):R1-R16. doi: 10.1152/ajpregu.00145.2019. Epub 2019 Oct 2. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 31577477 Free PMC article.
-
Uteroplacental Circulation in Normal Pregnancy and Preeclampsia: Functional Adaptation and Maladaptation.Int J Mol Sci. 2021 Aug 11;22(16):8622. doi: 10.3390/ijms22168622. Int J Mol Sci. 2021. PMID: 34445328 Free PMC article. Review.
-
Hypoxic modulation of fetal vascular MLCK abundance, localization, and function.Am J Physiol Regul Integr Comp Physiol. 2021 Jan 1;320(1):R1-R18. doi: 10.1152/ajpregu.00212.2020. Epub 2020 Oct 28. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 33112654 Free PMC article.
-
Long-term hypoxia modulates depolarization activation of BKCa currents in fetal sheep middle cerebral arterial myocytes.Front Physiol. 2024 Nov 5;15:1479882. doi: 10.3389/fphys.2024.1479882. eCollection 2024. Front Physiol. 2024. PMID: 39563935 Free PMC article.
References
-
- Angeles DM, Williams J, Zhang L, Pearce WJ. Acute hypoxia modulates 5-HT receptor density and agonist affinity in fetal and adult ovine carotid arteries. Am J Physiol Heart Circ Physiol 279: H502–H510, 2000. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

