Acute toll-like receptor 4 activation impairs rat renal microvascular autoregulatory behaviour
- PMID: 28544543
- PMCID: PMC5641241
- DOI: 10.1111/apha.12899
Acute toll-like receptor 4 activation impairs rat renal microvascular autoregulatory behaviour
Abstract
Aim: Little is known about how toll-like receptor 4 (TLR4) influences the renal microvasculature. We hypothesized that acute TLR4 stimulation with lipopolysaccharide (LPS) impairs afferent arteriole autoregulatory behaviour, partially through reactive oxygen species (ROS).
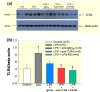
Methods: We assessed afferent arteriole autoregulatory behaviour after LPS treatment (1 mg kg-1 ; i.p.) using the in vitro blood-perfused juxtamedullary nephron preparation. Autoregulatory behaviour was assessed by measuring diameter responses to stepwise changes in renal perfusion pressure. TLR4 expression was assessed by immunofluorescence, immunohistochemistry and Western blot analysis in the renal cortex and vasculature.
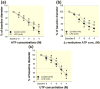
Results: Baseline arteriole diameter at 100 mmHg averaged 15.2 ± 1.2 μm and 12.2 ± 1.0 μm for control and LPS groups (P < 0.05) respectively. When perfusion pressure was increased in 15 mmHg increments from 65 to 170 mmHg, arteriole diameter in control kidneys decreased significantly to 69 ± 6% of baseline diameter. In the LPS-treated group, arteriole diameter remained essentially unchanged (103 ± 9% of baseline), indicating impaired autoregulatory behaviour. Pre-treatment with anti-TLR4 antibody or the TLR4 antagonist, LPS-RS, preserved autoregulatory behaviour during LPS treatment. P2 receptor reactivity was normal in control and LPS-treated rats. Pre-treatment with Losartan (angiotensin type 1 receptor blocker; (AT1 ) 2 mg kg-1 ; i.p.) increased baseline afferent arteriole diameter but did not preserve autoregulatory behaviour in LPS-treated rats. Acute exposure to Tempol (10-3 mol L-1 ), a superoxide dismutase mimetic, restored pressure-mediated vasoconstriction in kidneys from LPS-treated rats.
Conclusion: These data demonstrate that TLR4 activation impairs afferent arteriole autoregulatory behaviour, partially through ROS, but independently of P2 and AT1 receptor activation.
Keywords: endotoxemia; inflammation; oxidative stress; renal microcirculation; toll-like receptor 4.
© 2017 Scandinavian Physiological Society. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest in relation to the publication of the present study.
Figures

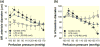
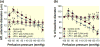
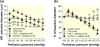

Similar articles
-
Antagonism of major histocompatibility complex class II invariant chain peptide during chronic lipopolysaccharide treatment rescues autoregulatory behavior.Am J Physiol Renal Physiol. 2019 Oct 1;317(4):F957-F966. doi: 10.1152/ajprenal.00164.2019. Epub 2019 Aug 21. Am J Physiol Renal Physiol. 2019. PMID: 31432707 Free PMC article.
-
Restoration of afferent arteriolar autoregulatory behavior in ischemia-reperfusion injury in rat kidneys.Am J Physiol Renal Physiol. 2021 Mar 1;320(3):F429-F441. doi: 10.1152/ajprenal.00500.2020. Epub 2021 Jan 25. Am J Physiol Renal Physiol. 2021. PMID: 33491564 Free PMC article.
-
Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats.J Am Soc Nephrol. 1999 Jan;10 Suppl 11:S178-83. J Am Soc Nephrol. 1999. PMID: 9892160
-
Afferent arteriole responsiveness to endothelin receptor activation: does sex matter?Biol Sex Differ. 2019 Jan 3;10(1):1. doi: 10.1186/s13293-018-0218-2. Biol Sex Differ. 2019. PMID: 30606254 Free PMC article.
-
Altered myogenic responsiveness of the renal microvasculature in experimental hypertension.J Hypertens. 1996 Dec;14(12):1387-401. doi: 10.1097/00004872-199612000-00002. J Hypertens. 1996. PMID: 8986920 Review.
Cited by
-
The renal vasodilatory effect of prostaglandins is ameliorated in isolated-perfused kidneys of endotoxemic mice.Pflugers Arch. 2018 Nov;470(11):1691-1703. doi: 10.1007/s00424-018-2183-3. Epub 2018 Jul 19. Pflugers Arch. 2018. PMID: 30027346
-
Antagonism of major histocompatibility complex class II invariant chain peptide during chronic lipopolysaccharide treatment rescues autoregulatory behavior.Am J Physiol Renal Physiol. 2019 Oct 1;317(4):F957-F966. doi: 10.1152/ajprenal.00164.2019. Epub 2019 Aug 21. Am J Physiol Renal Physiol. 2019. PMID: 31432707 Free PMC article.
-
Endothelial cells: potential novel regulators of renal inflammation.Am J Physiol Renal Physiol. 2022 Mar 1;322(3):F309-F321. doi: 10.1152/ajprenal.00371.2021. Epub 2022 Feb 7. Am J Physiol Renal Physiol. 2022. PMID: 35129369 Free PMC article. Review.
-
Cardiac and vascular complications in lupus: Is there a role for sex?Front Immunol. 2023 Mar 29;14:1098383. doi: 10.3389/fimmu.2023.1098383. eCollection 2023. Front Immunol. 2023. PMID: 37063843 Free PMC article. Review.
-
Role for ovarian hormones in purinoceptor-dependent natriuresis.Biol Sex Differ. 2020 Sep 14;11(1):52. doi: 10.1186/s13293-020-00329-0. Biol Sex Differ. 2020. PMID: 32928299 Free PMC article.
References
-
- Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol. 1978;234:F357–370. - PubMed
-
- Boffa JJ, Arendshorst WJ. Maintenance of renal vascular reactivity contributes to acute renal failure during endotoxemic shock. J Am Soc Nephrol. 2005;16:117–124. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

