The missing, the short, and the long: Levodopa responses and dopamine actions
- PMID: 28543679
- PMCID: PMC5526730
- DOI: 10.1002/ana.24961
The missing, the short, and the long: Levodopa responses and dopamine actions
Abstract
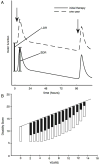
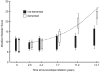
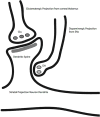
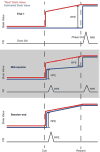
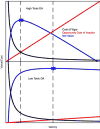
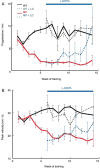
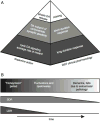
We attempt to correlate the clinical pharmacology of dopamine replacement therapy (DRT) in Parkinson Disease with known features of striatal dopamine actions. Despite its obvious impact, DRT does not normalize motor function, likely due to disrupted phasic dopaminergic signaling. The DRT Short Duration Response is likely a permissive-paracrine effect, possibly resulting from dopaminergic support of corticostriate synaptic plasticity. The DRT Long Duration Response may result from mimicry of tonic dopamine signaling regulation of movement vigor. Our understanding of dopamine actions does not explain important aspects of DRT clinical pharmacology. Reducing these knowledge gaps provides opportunities to improve understanding of dopamine actions and symptomatic treatment of Parkinson disease.
Conflict of interest statement
Potential Conflicts of Interest:
Neither Dr. Albin nor Dr. Leventhal have any financial relationships that could be perceived as a conflict of interest.
Figures
Comment in
-
Reply to "the missing, the short, and the long: Exploring the borderland between psychiatry and neurology".Ann Neurol. 2017 Sep;82(3):493-494. doi: 10.1002/ana.25033. Ann Neurol. 2017. PMID: 28856716 No abstract available.
-
The missing, the short, and the long: Exploring the borderland between psychiatry and neurology.Ann Neurol. 2017 Sep;82(3):493. doi: 10.1002/ana.25034. Ann Neurol. 2017. PMID: 28858398 No abstract available.
Similar articles
-
Did levodopa vs. dopamine agonist trials teach us when and how to start symptomatic therapy?Parkinsonism Relat Disord. 2009 Dec;15 Suppl 3:S31-3. doi: 10.1016/S1353-8020(09)70775-8. Parkinsonism Relat Disord. 2009. PMID: 20083002 No abstract available.
-
Agonists versus levodopa in PD: the thrilla of whitha.Neurology. 2003 Nov 25;61(10):1462; author reply 1462-3. doi: 10.1212/wnl.61.10.1462. Neurology. 2003. PMID: 14638991 No abstract available.
-
Imaging the dopamine system to assess disease-modifying drugs: studies comparing dopamine agonists and levodopa.Neurology. 2003 Sep 23;61(6 Suppl 3):S43-8. doi: 10.1212/wnl.61.6_suppl_3.s43. Neurology. 2003. PMID: 14504379 Review. No abstract available.
-
Dopamine agonists in early therapy for Parkinson disease: promise and problems.JAMA. 2000 Oct 18;284(15):1971-3. doi: 10.1001/jama.284.15.1971. JAMA. 2000. PMID: 11035895 No abstract available.
-
[Controversial issues concerning the initial treatment of Parkinson's disease: L-Dopa or dopaminergic agonists?].Rev Neurol (Paris). 1999 Jan;155(1):43-5. Rev Neurol (Paris). 1999. PMID: 10093847 Review. French. No abstract available.
Cited by
-
Adopting the Rumsfeld approach to understanding the action of levodopa and apomorphine in Parkinson's disease.J Neural Transm (Vienna). 2023 Nov;130(11):1337-1347. doi: 10.1007/s00702-023-02655-0. Epub 2023 May 20. J Neural Transm (Vienna). 2023. PMID: 37210460 Free PMC article. Review.
-
Neuroplasticity in Parkinson's disease.J Neural Transm (Vienna). 2024 Nov;131(11):1329-1339. doi: 10.1007/s00702-024-02813-y. Epub 2024 Aug 5. J Neural Transm (Vienna). 2024. PMID: 39102007 Free PMC article. Review.
-
Tourette Syndrome as a Disorder of the Social Decision Making Network.Front Psychiatry. 2019 Oct 8;10:742. doi: 10.3389/fpsyt.2019.00742. eCollection 2019. Front Psychiatry. 2019. PMID: 31649568 Free PMC article. Review.
-
Current Knowledge on the Background, Pathophysiology and Treatment of Levodopa-Induced Dyskinesia-Literature Review.J Clin Med. 2021 Sep 25;10(19):4377. doi: 10.3390/jcm10194377. J Clin Med. 2021. PMID: 34640395 Free PMC article. Review.
-
Normalization effect of dopamine replacement therapy on brain functional connectome in Parkinson's disease.Hum Brain Mapp. 2023 Jun 15;44(9):3845-3858. doi: 10.1002/hbm.26316. Epub 2023 May 1. Hum Brain Mapp. 2023. PMID: 37126590 Free PMC article.
References
-
- Schultz W. Multiple Dopamine Functions at Different Time Courses. Ann Rev Neurosci. 2007;30:259–288. - PubMed
-
- Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. New Eng J Med. 2004;351:2498–2508. - PubMed
-
- Cotzias GC, Van Woert MH, Schiffer LM. Aromatic amino acids and modification of parkinsonism. N Engl J Med. 1967;276:374–379. - PubMed
-
- Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism–chronic treatment with L-dopa. N Engl J Med. 1969;280:337–345. - PubMed
-
- Muenter MD, Tyce GM. L-dopa therapy of Parkinson’s disease: plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clin Proc. 1971;46:231–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

