Pioneer neurog1 expressing cells ingress into the otic epithelium and instruct neuronal specification
- PMID: 28537554
- PMCID: PMC5476427
- DOI: 10.7554/eLife.25543
Pioneer neurog1 expressing cells ingress into the otic epithelium and instruct neuronal specification
Abstract
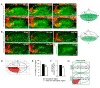
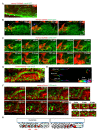
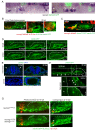
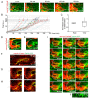
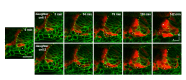
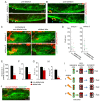
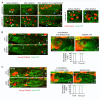
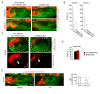
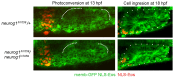
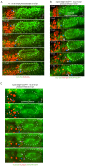
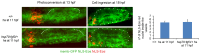
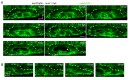
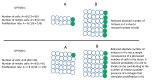
Neural patterning involves regionalised cell specification. Recent studies indicate that cell dynamics play instrumental roles in neural pattern refinement and progression, but the impact of cell behaviour and morphogenesis on neural specification is not understood. Here we combine 4D analysis of cell behaviours with dynamic quantification of proneural expression to uncover the construction of the zebrafish otic neurogenic domain. We identify pioneer cells expressing neurog1 outside the otic epithelium that migrate and ingress into the epithelialising placode to become the first otic neuronal progenitors. Subsequently, neighbouring cells express neurog1 inside the placode, and apical symmetric divisions amplify the specified pool. Interestingly, pioneer cells delaminate shortly after ingression. Ablation experiments reveal that pioneer cells promote neurog1 expression in other otic cells. Finally, ingression relies on the epithelialisation timing controlled by FGF activity. We propose a novel view for otic neurogenesis integrating cell dynamics whereby ingression of pioneer cells instructs neuronal specification.
Keywords: FGF; cell dynamics; developmental biology; morphogenesis; neurog1; neuronal specification; neuroscience; stem cells; zebrafish.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
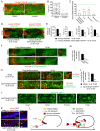
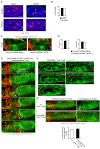
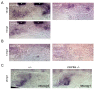
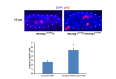
Similar articles
-
SOX2 is required for inner ear neurogenesis.Sci Rep. 2017 Jun 22;7(1):4086. doi: 10.1038/s41598-017-04315-2. Sci Rep. 2017. PMID: 28642583 Free PMC article.
-
NEUROG1 Regulates CDK2 to Promote Proliferation in Otic Progenitors.Stem Cell Reports. 2017 Nov 14;9(5):1516-1529. doi: 10.1016/j.stemcr.2017.09.011. Epub 2017 Oct 12. Stem Cell Reports. 2017. PMID: 29033307 Free PMC article.
-
Zebrafish Foxi1 provides a neuronal ground state during inner ear induction preceding the Dlx3b/4b-regulated sensory lineage.Development. 2013 May;140(9):1936-45. doi: 10.1242/dev.087718. Development. 2013. PMID: 23571216
-
Signaling mechanisms controlling cranial placode neurogenesis and delamination.Dev Biol. 2014 May 1;389(1):39-49. doi: 10.1016/j.ydbio.2013.11.025. Epub 2013 Dec 3. Dev Biol. 2014. PMID: 24315854 Free PMC article. Review.
-
Neurogenesis in zebrafish embryos.Methods Cell Biol. 1999;59:367-86. doi: 10.1016/s0091-679x(08)61835-x. Methods Cell Biol. 1999. PMID: 9891370 Review. No abstract available.
Cited by
-
shox2 is required for vestibular statoacoustic neuron development.Biol Open. 2022 Dec 15;11(12):bio059599. doi: 10.1242/bio.059599. Epub 2022 Dec 21. Biol Open. 2022. PMID: 36594417 Free PMC article.
-
A new mode of pancreatic islet innervation revealed by live imaging in zebrafish.Elife. 2018 Jun 19;7:e34519. doi: 10.7554/eLife.34519. Elife. 2018. PMID: 29916364 Free PMC article.
-
Pioneer statoacoustic neurons guide neuroblast behaviour during otic ganglion assembly.Development. 2023 Nov 1;150(21):dev201824. doi: 10.1242/dev.201824. Epub 2023 Nov 8. Development. 2023. PMID: 37938828 Free PMC article.
-
Anatomical map of the cranial vasculature and sensory ganglia.J Anat. 2018 Mar;232(3):431-439. doi: 10.1111/joa.12762. Epub 2017 Dec 13. J Anat. 2018. PMID: 29235648 Free PMC article.
-
Nomo1 deficiency causes autism-like behavior in zebrafish.EMBO Rep. 2024 Feb;25(2):570-592. doi: 10.1038/s44319-023-00036-y. Epub 2024 Jan 22. EMBO Rep. 2024. PMID: 38253686 Free PMC article.
References
-
- Abelló G, Khatri S, Radosevic M, Scotting PJ, Giráldez F, Alsina B. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Developmental Biology. 2010;339:166–178. doi: 10.1016/j.ydbio.2009.12.027. - DOI - PubMed
-
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

