WNT5A-ROR2 is induced by inflammatory mediators and is involved in the migration of human ovarian cancer cell line SKOV-3
- PMID: 28536612
- PMCID: PMC5415827
- DOI: 10.1186/s11658-016-0003-3
WNT5A-ROR2 is induced by inflammatory mediators and is involved in the migration of human ovarian cancer cell line SKOV-3
Abstract
Background: Wnt5A, which is a member of the non-transforming Wnt protein family, is implicated in inflammatory processes. It is also highly expressed by ovarian cancer cells. ROR2, which is a member of the Ror-family of receptor tyrosine kinases, acts as a receptor or co-receptor for Wnt5A. The Wnt5A-ROR2 signaling pathway plays essential roles in the migration and invasion of several types of tumor cell and influences their cell polarity. We investigated the modulation of Wnt5A-ROR2 by inflammatory mediators and its involvement in the migration of the human ovarian cancer cell line SKOV-3.
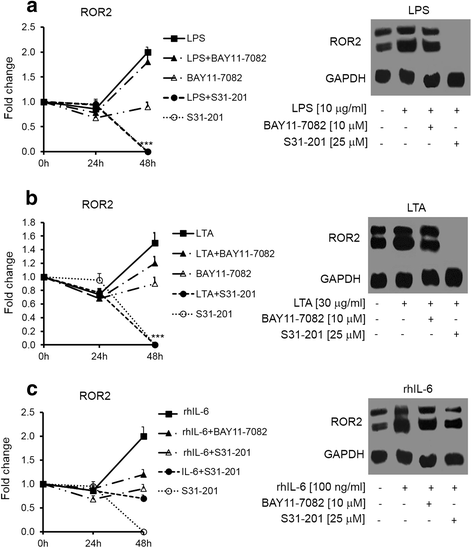
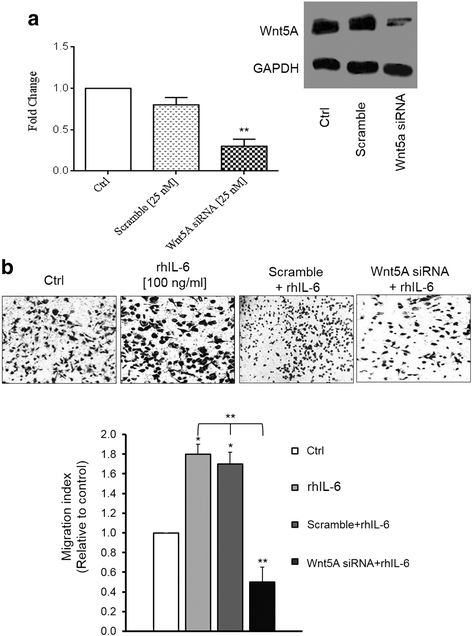
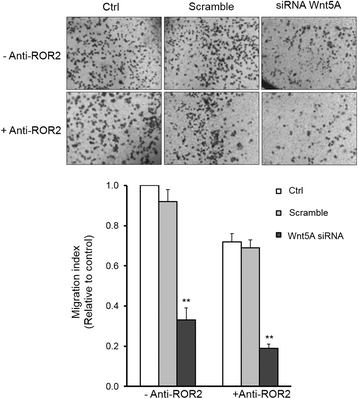
Methods: SKOV-3 cells were treated with LPS (lipopolysaccharide), LTA (lipoteichoic acid) and recombinant human IL-6 alone or in combination with STAT3 inhibitor (S1155S31-201) or NF-kB inhibitor (BAY11-7082) for 4, 8, 12, 24 and 48 h. The Wnt5A and ROR2 expression levels were determined at the gene and protein levels. Cells were transfected with specific siRNA against Wnt5A in the absence or presence of human anti-ROR2 antibody and cell migration was assessed using transwells.
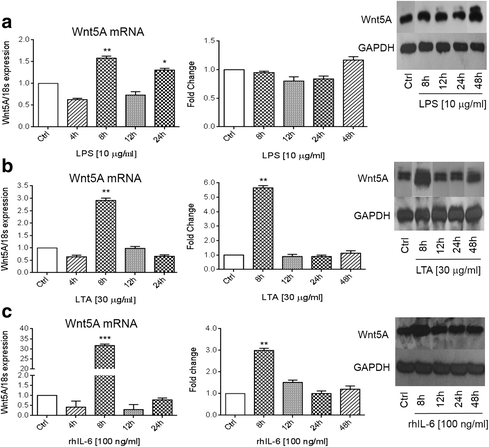
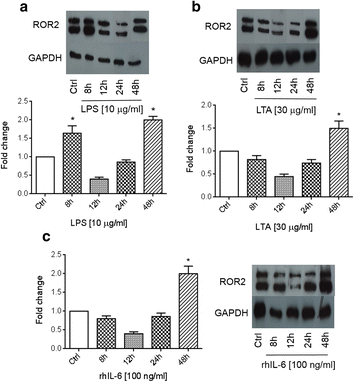
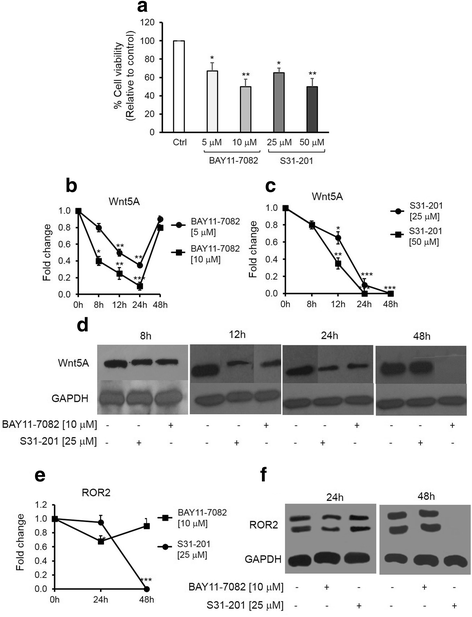
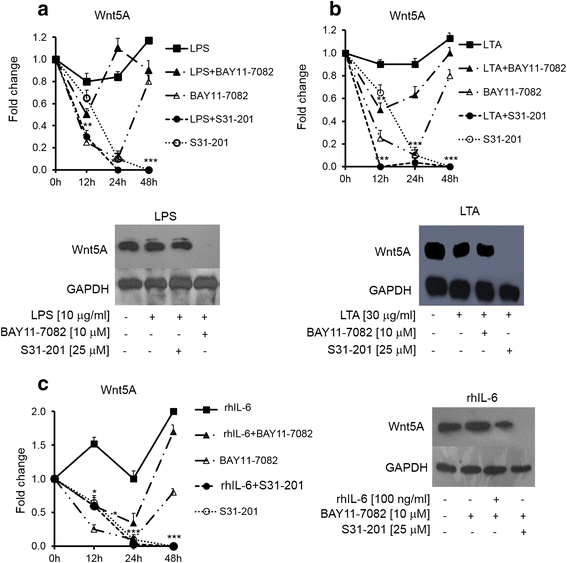
Results: There was a strong downregulation of Wnt5A expression in the presence of STAT3 or NF-kB inhibitors. Cell stimulation with LTA or IL-6 for 8 h led to significantly increased levels of Wnt5A (5- and 3-fold higher, respectively). LPS, LTA or IL-6 treatment significantly increased ROR2 expression (2-fold after 48 h). LPS- or LTA-induced Wnt5A or ROR2 expression was abrogated in the presence of STAT3 inhibitor (p < 0.001). IL-6-induced Wnt5A expression was abrogated by both STAT3 and NF-kB inhibitors (p < 0.001). Although not significant, IL-6-induced ROR2 expression showed a modest decrease when STAT3 inhibitor was used. Moreover, cell migration was decreased by 80 % in siRNA Wnt5A-transfected cells in the presence of anti-human ROR2 antibody (p < 0.001).
Conclusions: This study revealed for the first time that inflammatory mediators modulate Wnt5A and ROR2 through NF-kB and STAT3 transcription factors and this may play a role in ovarian cancer cell migration. The results described here provide new insight into the role of the Wnt5A-ROR2 complex in ovarian cancer progression in relation to inflammation.
Keywords: Inflammation; Migration; NF-kB/STAT3 signaling pathways; Ovarian cancer; ROR2; Wnt5A.
Figures
Similar articles
-
Role of the Ror family receptors in Wnt5a signaling.In Vitro Cell Dev Biol Anim. 2024 May;60(5):489-501. doi: 10.1007/s11626-024-00885-4. Epub 2024 Apr 8. In Vitro Cell Dev Biol Anim. 2024. PMID: 38587578 Review.
-
Wnt5A exerts immunomodulatory activity in the human ovarian cancer cell line SKOV-3.Cell Biol Int. 2016 Feb;40(2):177-87. doi: 10.1002/cbin.10551. Epub 2015 Nov 9. Cell Biol Int. 2016. PMID: 26462870
-
Wnt5A regulates the expression of ROR2 tyrosine kinase receptor in ovarian cancer cells.Biochem Cell Biol. 2017 Dec;95(6):609-615. doi: 10.1139/bcb-2016-0216. Epub 2017 May 24. Biochem Cell Biol. 2017. Retraction in: Biochem Cell Biol. 2023 Jun 1;101(3):268. doi: 10.1139/bcb-2023-0066. PMID: 28538104 Retracted.
-
Targeting the ROR1 and ROR2 receptors in epithelial ovarian cancer inhibits cell migration and invasion.Oncotarget. 2015 Nov 24;6(37):40310-26. doi: 10.18632/oncotarget.5643. Oncotarget. 2015. PMID: 26515598 Free PMC article.
-
Insight into the role of Wnt5a-induced signaling in normal and cancer cells.Int Rev Cell Mol Biol. 2015;314:117-48. doi: 10.1016/bs.ircmb.2014.10.003. Epub 2014 Nov 18. Int Rev Cell Mol Biol. 2015. PMID: 25619716 Review.
Cited by
-
Postbiotics as Adjuvant Therapy in Cancer Care.Nutrients. 2024 Jul 24;16(15):2400. doi: 10.3390/nu16152400. Nutrients. 2024. PMID: 39125280 Free PMC article. Review.
-
Role of the Ror family receptors in Wnt5a signaling.In Vitro Cell Dev Biol Anim. 2024 May;60(5):489-501. doi: 10.1007/s11626-024-00885-4. Epub 2024 Apr 8. In Vitro Cell Dev Biol Anim. 2024. PMID: 38587578 Review.
-
Satureja bachtiarica Induces Cancer Cell Death in Breast and Glioblastoma Cancer in 2D/3D Models and Suppresses Breast Cancer Stem Cells.Cells. 2023 Nov 26;12(23):2713. doi: 10.3390/cells12232713. Cells. 2023. PMID: 38067141 Free PMC article.
-
Wnt signaling regulates ion channel expression to promote smooth muscle and cartilage formation in developing mouse trachea.Am J Physiol Lung Cell Mol Physiol. 2023 Dec 1;325(6):L788-L802. doi: 10.1152/ajplung.00024.2023. Epub 2023 Oct 24. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 37873566
-
Postbiotics in oncology: science or science fiction?Front Microbiol. 2023 Aug 7;14:1182547. doi: 10.3389/fmicb.2023.1182547. eCollection 2023. Front Microbiol. 2023. PMID: 37608943 Free PMC article. Review.
References
-
- World Cancer Report 2014. International Agency for Research on Cancer
-
- Minami Y, Oishi I, Endo M, Nishita M. Ror family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev Dyn. 2010;239:1–15. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

