Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures
- PMID: 28521131
- PMCID: PMC5523817
- DOI: 10.1016/j.neuron.2017.04.043
Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures
Abstract
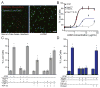
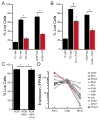
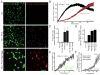
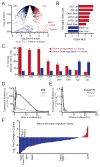
Microglia, the resident macrophages of the CNS, engage in various CNS-specific functions that are critical for development and health. To better study microglia and the properties that distinguish them from other tissue macrophage populations, we have optimized serum-free culture conditions to permit robust survival of highly ramified adult microglia under defined-medium conditions. We find that astrocyte-derived factors prevent microglial death ex vivo and that this activity results from three primary components, CSF-1/IL-34, TGF-β2, and cholesterol. Using microglial cultures that have never been exposed to serum, we demonstrate a dramatic and lasting change in phagocytic capacity after serum exposure. Finally, we find that mature microglia rapidly lose signature gene expression after isolation, and that this loss can be reversed by engrafting cells back into an intact CNS environment. These data indicate that the specialized gene expression profile of mature microglia requires continuous instructive signaling from the intact CNS.
Keywords: CSF1R; TGF-β; astrocyte; cholesterol; inflammation; maturation; microglia; neurodegeneration; phagocytosis; transplant.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production.J Neurosci Res. 1998 Oct 1;54(1):68-78. doi: 10.1002/(SICI)1097-4547(19981001)54:1<68::AID-JNR8>3.0.CO;2-F. J Neurosci Res. 1998. PMID: 9778151
-
Isolation and Culture of Rodent Microglia to Promote a Dynamic Ramified Morphology in Serum-free Medium.J Vis Exp. 2018 Mar 9;(133):57122. doi: 10.3791/57122. J Vis Exp. 2018. PMID: 29578519 Free PMC article.
-
Pigment epithelium-derived factor (PEDF) has direct effects on the metabolism and proliferation of microglia and indirect effects on astrocytes.J Neurosci Res. 1997 Sep 15;49(6):710-8. doi: 10.1002/(SICI)1097-4547(19970915)49:6<710::AID-JNR5>3.0.CO;2-A. J Neurosci Res. 1997. PMID: 9335258
-
Microglia Development and Maturation and Its Implications for Induction of Microglia-Like Cells from Human iPSCs.Int J Mol Sci. 2021 Mar 17;22(6):3088. doi: 10.3390/ijms22063088. Int J Mol Sci. 2021. PMID: 33803024 Free PMC article. Review.
-
Core Concept: Cells nibble one another via the under-appreciated process of trogocytosis.Proc Natl Acad Sci U S A. 2019 Sep 3;116(36):17608-17610. doi: 10.1073/pnas.1912252116. Proc Natl Acad Sci U S A. 2019. PMID: 31481628 Free PMC article. Review. No abstract available.
Cited by
-
Microglia contribute to neuronal synchrony despite endogenous ATP-related phenotypic transformation in acute mouse brain slices.Nat Commun. 2024 Jun 26;15(1):5402. doi: 10.1038/s41467-024-49773-1. Nat Commun. 2024. PMID: 38926390 Free PMC article.
-
The appearance of phagocytic microglia in the postnatal brain of Niemann Pick type C mice is developmentally regulated and underscores shortfalls in fine odor discrimination.J Cell Physiol. 2022 Dec;237(12):4563-4579. doi: 10.1002/jcp.30909. Epub 2022 Nov 2. J Cell Physiol. 2022. PMID: 36322609 Free PMC article.
-
When glia meet induced pluripotent stem cells (iPSCs).Mol Cell Neurosci. 2020 Dec;109:103565. doi: 10.1016/j.mcn.2020.103565. Epub 2020 Oct 14. Mol Cell Neurosci. 2020. PMID: 33068719 Free PMC article. Review.
-
From Physiology to Pathology of Astrocytes: Highlighting Their Potential as Therapeutic Targets for CNS Injury.Neurosci Bull. 2025 Jan;41(1):131-154. doi: 10.1007/s12264-024-01258-3. Epub 2024 Jul 30. Neurosci Bull. 2025. PMID: 39080102 Free PMC article. Review.
-
β-Hydroxybutyrate Improves the Redox Status, Cytokine Production and Phagocytic Potency of Glucose-Deprived HMC3 Human Microglia-like Cells.J Neuroimmune Pharmacol. 2024 Jul 23;19(1):35. doi: 10.1007/s11481-024-10139-5. J Neuroimmune Pharmacol. 2024. PMID: 39042253
References
-
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. - PubMed
-
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. - PubMed
-
- Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–216. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

