The alphabet of intrinsic disorder: II. Various roles of glutamic acid in ordered and intrinsically disordered proteins
- PMID: 28516010
- PMCID: PMC5424795
- DOI: 10.4161/idp.24684
The alphabet of intrinsic disorder: II. Various roles of glutamic acid in ordered and intrinsically disordered proteins
Abstract
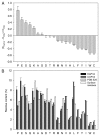
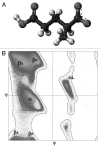
The ability of a protein to fold into unique functional state or to stay intrinsically disordered is encoded in its amino acid sequence. Both ordered and intrinsically disordered proteins (IDPs) are natural polypeptides that use the same arsenal of 20 proteinogenic amino acid residues as their major building blocks. The exceptional structural plasticity of IDPs, their capability to exist as heterogeneous structural ensembles and their wide array of important disorder-based biological functions that complements functional repertoire of ordered proteins are all rooted within the peculiar differential usage of these building blocks by ordered proteins and IDPs. In fact, some residues (so-called disorder-promoting residues) are noticeably more common in IDPs than in sequences of ordered proteins, which, in their turn, are enriched in several order-promoting residues. Furthermore, residues can be arranged according to their "disorder promoting potencies," which are evaluated based on the relative abundances of various amino acids in ordered and disordered proteins. This review continues a series of publications on the roles of different amino acids in defining the phenomenon of protein intrinsic disorder and concerns glutamic acid, which is the second most disorder-promoting residue.
Keywords: glutamic acid; intrinsically disordered protein; protein function; protein structure; protein-protein interaction.
Figures
Similar articles
-
The intrinsic disorder alphabet. III. Dual personality of serine.Intrinsically Disord Proteins. 2015 Mar 17;3(1):e1027032. doi: 10.1080/21690707.2015.1027032. eCollection 2015. Intrinsically Disord Proteins. 2015. PMID: 28232888 Free PMC article. Review.
-
The alphabet of intrinsic disorder: I. Act like a Pro: On the abundance and roles of proline residues in intrinsically disordered proteins.Intrinsically Disord Proteins. 2013 Apr 1;1(1):e24360. doi: 10.4161/idp.24360. eCollection 2013 Jan-Dec. Intrinsically Disord Proteins. 2013. PMID: 28516008 Free PMC article. Review.
-
Do sequence neighbours of intrinsically disordered regions promote structural flexibility in intrinsically disordered proteins?J Struct Biol. 2020 Feb 1;209(2):107428. doi: 10.1016/j.jsb.2019.107428. Epub 2019 Nov 20. J Struct Biol. 2020. PMID: 31756456
-
Functional unfoldomics: Roles of intrinsic disorder in protein (multi)functionality.Adv Protein Chem Struct Biol. 2024;138:179-210. doi: 10.1016/bs.apcsb.2023.11.001. Epub 2023 Nov 22. Adv Protein Chem Struct Biol. 2024. PMID: 38220424 Review.
-
Self-assembling systems comprising intrinsically disordered protein polymers like elastin-like recombinamers.J Pept Sci. 2022 Jan;28(1):e3362. doi: 10.1002/psc.3362. Epub 2021 Sep 20. J Pept Sci. 2022. PMID: 34545666 Review.
Cited by
-
Bioinformatic Analysis and Biophysical Characterization Reveal Structural Disorder in G0S2 Protein.ACS Omega. 2020 Oct 5;5(40):25841-25847. doi: 10.1021/acsomega.0c03171. eCollection 2020 Oct 13. ACS Omega. 2020. PMID: 33073109 Free PMC article.
-
Fluorinated Protein and Peptide Materials for Biomedical Applications.Pharmaceuticals (Basel). 2022 Sep 28;15(10):1201. doi: 10.3390/ph15101201. Pharmaceuticals (Basel). 2022. PMID: 36297312 Free PMC article. Review.
-
The unfoldase ClpC1 of Mycobacterium tuberculosis regulates the expression of a distinct subset of proteins having intrinsically disordered termini.J Biol Chem. 2020 Jul 10;295(28):9455-9473. doi: 10.1074/jbc.RA120.013456. Epub 2020 May 14. J Biol Chem. 2020. PMID: 32409584 Free PMC article.
-
Protein structure-function continuum model: Emerging nexuses between specificity, evolution, and structure.Protein Sci. 2024 Apr;33(4):e4968. doi: 10.1002/pro.4968. Protein Sci. 2024. PMID: 38532700 Review.
-
Disordered Antigens and Epitope Overlap Between Anti-Citrullinated Protein Antibodies and Rheumatoid Factor in Rheumatoid Arthritis.Arthritis Rheumatol. 2020 Feb;72(2):262-272. doi: 10.1002/art.41074. Epub 2019 Dec 10. Arthritis Rheumatol. 2020. PMID: 31397047 Free PMC article.
References
-
- Uversky VN. . Intrinsically disordered proteins from A to Z. Int J Biochem Cell Biol 2011; 43:1090 - 103; http://dx.doi.org/10.1016/j.biocel.2011.04.001; PMID: 21501695 - DOI - PubMed
-
- Tompa P. . Unstructural biology coming of age. Curr Opin Struct Biol 2011; 21:419 - 25; http://dx.doi.org/10.1016/j.sbi.2011.03.012; PMID: 21514142 - DOI - PubMed
-
- Romero P, Obradovic Z, Kissinger CR, Villafranca JE, Garner E, Guilliot S, et al. . . Thousands of proteins likely to have long disordered regions. Pac Symp Biocomput 1998; •••:437 - 48; PMID: 9697202 - PubMed
-
- Dunker AK, Obradovic Z, Romero P, Garner EC, Brown CJ. . Intrinsic protein disorder in complete genomes. Genome Inform Ser Workshop Genome Inform 2000; 11:161 - 71; PMID: 11700597 - PubMed
-
- Uversky VN, Gillespie JR, Fink AL. . Why are “natively unfolded” proteins unstructured under physiologic conditions?. Proteins 2000; 41:415 - 27; http://dx.doi.org/10.1002/1097-0134(20001115)41:3<415::AID-PROT130>3.0.CO;2-7; PMID: 11025552 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
