A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines
- PMID: 28515942
- PMCID: PMC5433150
- DOI: 10.1186/s40425-017-0246-1
A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines
Abstract
Background: Clinical success with chimeric antigen receptor (CAR)- based immunotherapy for leukemia has been accompanied by the associated finding that antigen-escape variants of the disease are responsible for relapse. To target hematologic malignancies with a chimeric antigen receptor (CAR) that targets two antigens with a single vector, and thus potentially lessen the chance of leukemic escape mutations, a tandem-CAR approach was investigated.
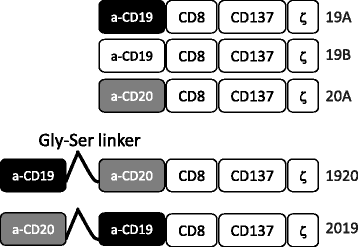
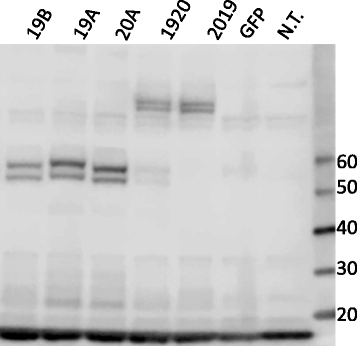
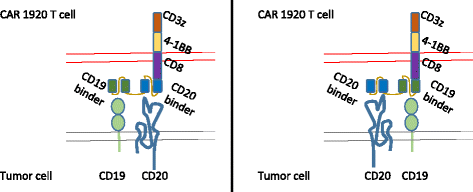
Methods: Antigen binding domains from the FMC63 (anti-CD19) and Leu16 (anti-CD20) antibodies were linked in differing configurations to transmembrane and T cell signaling domains to create tandem-CARs. Expression on the surface of primary human T cells was induced by transduction with a single lentiviral vector (LV) encoding the tandem-CAR. Tandem-CARs were compared to single antigen targeting CARs in vitro and in vivo, and to an admixture of transduced cells expressing each CAR in vivo in immunodeficient (NSG) disease-bearing mice.
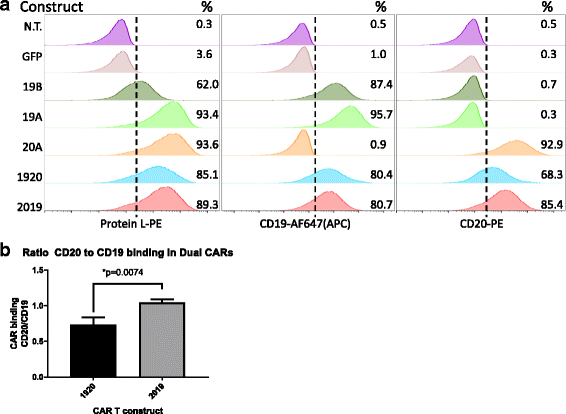
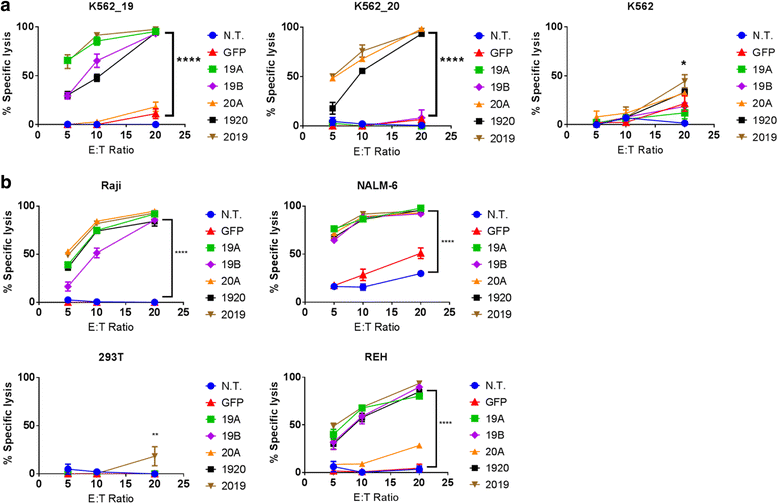
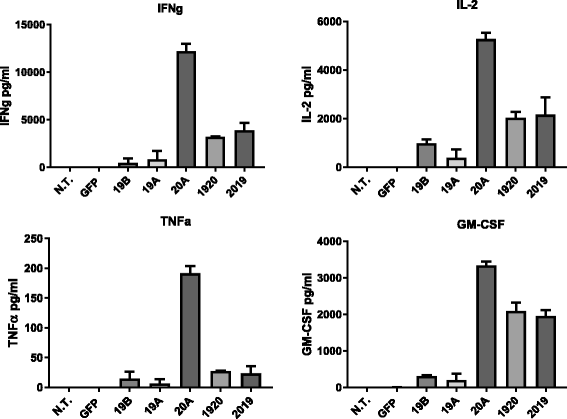
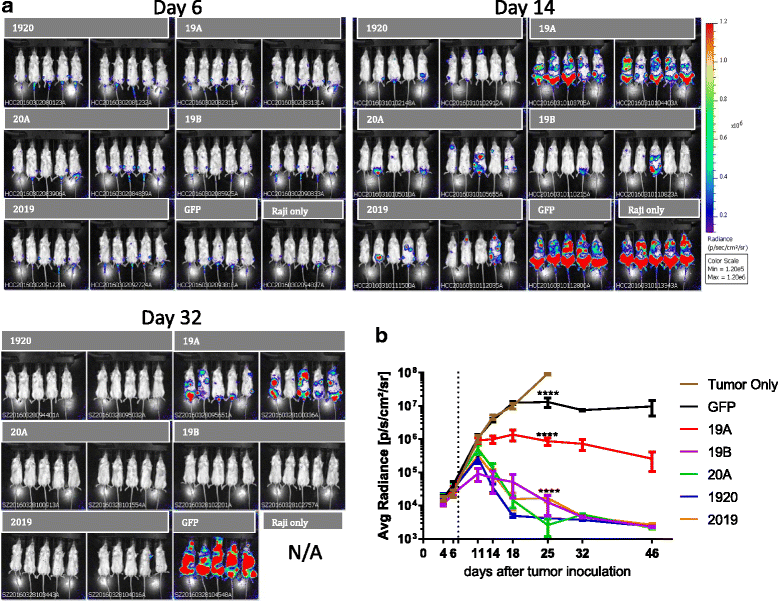
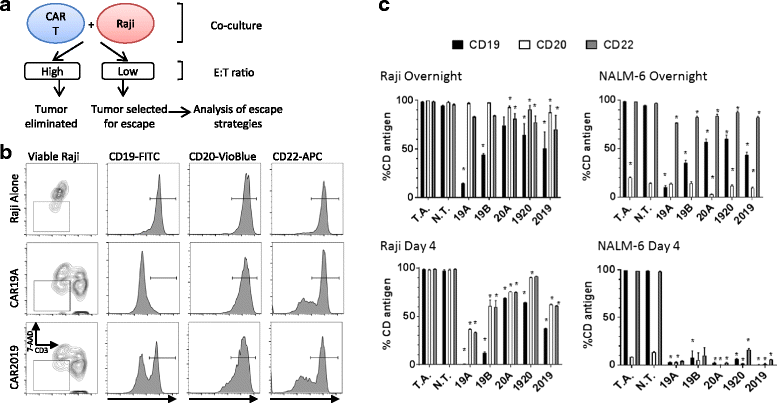
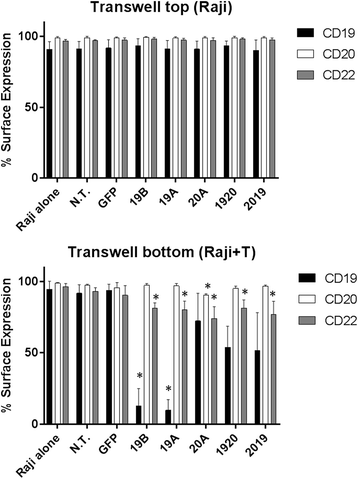
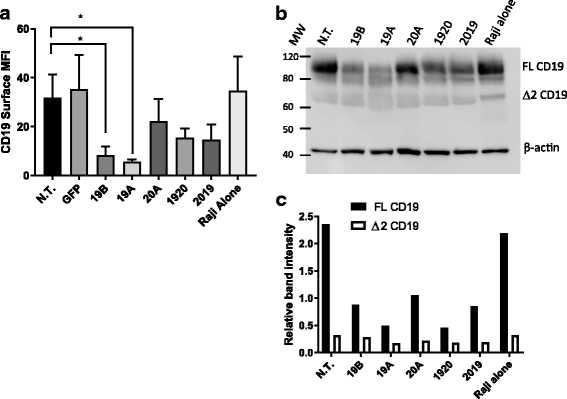
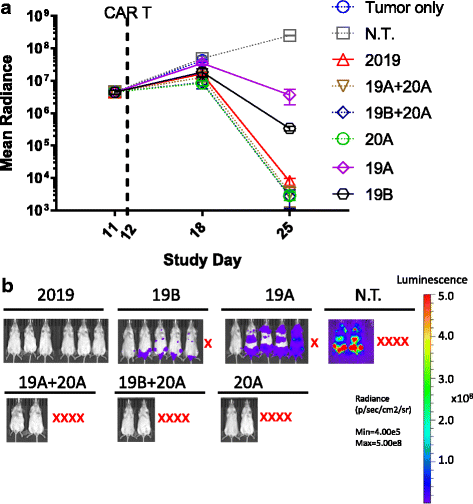
Results: Tandem constructs efficient killed the Raji leukemia cell line both in vitro and in vivo. Tandem CARs generated less cytokine than the CD20 CAR, but similar to CD19 CARs, on their own. In co-culture experiments at low effector to target ratios with both single- and tandem- CAR-T cells, a rapid down-modulation of full-length CD19 expression was seen on leukemia targets. There also was a partial down-modulation of CD22, and to a lesser degree, of CD20. Our data also highlight the extreme sensitivity of the NALM-6 cell line to general lymphocyte-mediated cytotoxicity. While single and tandem constructs were effective in vivo in a standard setting, in a high-disease burden setting, the tandem CAR proved both effective and less toxic than an admixture of transduced T cell populations expressing single CARs.
Conclusion: Tandem CARs are equally effective in standard disease models to single antigen specificity CARs, and may be both more effective and less toxic in a higher disease burden setting. This may be due to optimized cell killing with more moderate cytokine production. The rapid co-modulation of CD19, CD20, and CD22 may account for the ability to rapidly evolve escape mutants by selecting for leukemic clones that not require these target antigens for continued expansion.
Keywords: Adoptive immunotherapy; CAR T; CD19; CD20; CD22; Hematologic malignancy; Lentiviral vector; Tandem -targeting CAR; Tumor antigen escape.
Figures
Similar articles
-
T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells.Cancer Immunol Res. 2016 Jun;4(6):498-508. doi: 10.1158/2326-6066.CIR-15-0231. Epub 2016 Apr 8. Cancer Immunol Res. 2016. PMID: 27059623 Free PMC article.
-
Development of a compact bidirectional promoter-driven dual chimeric antigen receptor (CAR) construct targeting CD19 and CD20 in the Sleeping Beauty (SB) transposon system.J Immunother Cancer. 2024 Apr 27;12(4):e008555. doi: 10.1136/jitc-2023-008555. J Immunother Cancer. 2024. PMID: 38677881 Free PMC article.
-
CD20-CD19 Bispecific CAR T Cells for the Treatment of B-Cell Malignancies.Hum Gene Ther. 2017 Dec;28(12):1147-1157. doi: 10.1089/hum.2017.126. Hum Gene Ther. 2017. PMID: 29207878
-
Sequential anti-CD19, 22, and 20 autologous chimeric antigen receptor T-cell (CAR-T) treatments of a child with relapsed refractory Burkitt lymphoma: a case report and literature review.J Cancer Res Clin Oncol. 2020 Jun;146(6):1575-1582. doi: 10.1007/s00432-020-03198-7. Epub 2020 Mar 28. J Cancer Res Clin Oncol. 2020. PMID: 32222815 Review.
-
In Like a Lamb; Out Like a Lion: Marching CAR T Cells Toward Enhanced Efficacy in B-ALL.Mol Cancer Ther. 2021 Jul;20(7):1223-1233. doi: 10.1158/1535-7163.MCT-20-1089. Epub 2021 Apr 26. Mol Cancer Ther. 2021. PMID: 33903140 Free PMC article. Review.
Cited by
-
Industrializing engineered autologous T cells as medicines for solid tumours.Nat Rev Drug Discov. 2021 Jun;20(6):476-488. doi: 10.1038/s41573-021-00175-8. Epub 2021 Apr 8. Nat Rev Drug Discov. 2021. PMID: 33833444 Review.
-
Dawn of Chimeric Antigen Receptor T Cell Therapy in Non-Hodgkin Lymphoma.Adv Cell Gene Ther. 2018 Nov;1(3):e23. doi: 10.1002/acg2.23. Epub 2018 Oct 7. Adv Cell Gene Ther. 2018. PMID: 33043278 Free PMC article.
-
PD-1 blockade after bispecific LV20.19 CAR T modulates CAR T-cell immunophenotype without meaningful clinical response.Haematologica. 2021 Oct 1;106(10):2788-2790. doi: 10.3324/haematol.2021.278461. Haematologica. 2021. PMID: 33853295 Free PMC article. No abstract available.
-
Combined targeting of soluble latent TGF-ß and a solid tumor-associated antigen with adapter CAR T cells.Oncoimmunology. 2022 Nov 11;11(1):2140534. doi: 10.1080/2162402X.2022.2140534. eCollection 2022. Oncoimmunology. 2022. PMID: 36387056 Free PMC article.
-
Advances in immunotherapeutic targets for childhood cancers: A focus on glypican-2 and B7-H3.Pharmacol Ther. 2021 Jul;223:107892. doi: 10.1016/j.pharmthera.2021.107892. Epub 2021 May 14. Pharmacol Ther. 2021. PMID: 33992682 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
