Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis
- PMID: 28514601
- PMCID: PMC5548295
- DOI: 10.1056/NEJMoa1702079
Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis
Abstract
Background: Eosinophilic granulomatosis with polyangiitis is an eosinophilic vasculitis. Mepolizumab, an anti-interleukin-5 monoclonal antibody, reduces blood eosinophil counts and may have value in the treatment of eosinophilic granulomatosis with polyangiitis.
Methods: In this multicenter, double-blind, parallel-group, phase 3 trial, we randomly assigned participants with relapsing or refractory eosinophilic granulomatosis with polyangiitis who had received treatment for at least 4 weeks and were taking a stable prednisolone or prednisone dose to receive 300 mg of mepolizumab or placebo, administered subcutaneously every 4 weeks, plus standard care, for 52 weeks. The two primary end points were the accrued weeks of remission over a 52-week period, according to categorical quantification, and the proportion of participants in remission at both week 36 and week 48. Secondary end points included the time to first relapse and the average daily glucocorticoid dose (during weeks 48 through 52). The annualized relapse rate and safety were assessed.
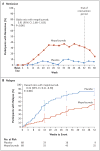
Results: A total of 136 participants underwent randomization, with 68 participants assigned to receive mepolizumab and 68 to receive placebo. Mepolizumab treatment led to significantly more accrued weeks of remission than placebo (28% vs. 3% of the participants had ≥24 weeks of accrued remission; odds ratio, 5.91; 95% confidence interval [CI], 2.68 to 13.03; P<0.001) and a higher percentage of participants in remission at both week 36 and week 48 (32% vs. 3%; odds ratio, 16.74; 95% CI, 3.61 to 77.56; P<0.001). Remission did not occur in 47% of the participants in the mepolizumab group versus 81% of those in the placebo group. The annualized relapse rate was 1.14 in the mepolizumab group, as compared with 2.27 in the placebo group (rate ratio, 0.50; 95% CI, 0.36 to 0.70; P<0.001). A total of 44% of the participants in the mepolizumab group, as compared with 7% of those in the placebo group, had an average daily dose of prednisolone or prednisone of 4.0 mg or less per day during weeks 48 through 52 (odds ratio, 0.20; 95% CI, 0.09 to 0.41; P<0.001). The safety profile of mepolizumab was similar to that observed in previous studies.
Conclusions: In participants with eosinophilic granulomatosis with polyangiitis, mepolizumab resulted in significantly more weeks in remission and a higher proportion of participants in remission than did placebo, thus allowing for reduced glucocorticoid use. Even so, only approximately half the participants treated with mepolizumab had protocol-defined remission. (Funded by GlaxoSmithKline and the National Institute of Allergy and Infectious Diseases; ClinicalTrials.gov number, NCT02020889 .).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Vasculitis: Mepolizumab for eosinophilic granulomatosis with polyangiitis.Nat Rev Rheumatol. 2017 Sep;13(9):518-519. doi: 10.1038/nrrheum.2017.117. Epub 2017 Aug 3. Nat Rev Rheumatol. 2017. PMID: 28769112 No abstract available.
-
Novel Treatments for Airway Disease.N Engl J Med. 2017 Aug 10;377(6):597. doi: 10.1056/NEJMc1708004. N Engl J Med. 2017. PMID: 28792871 No abstract available.
-
Novel Treatments for Airway Disease.N Engl J Med. 2017 Aug 10;377(6):596-7. doi: 10.1056/NEJMc1708004. N Engl J Med. 2017. PMID: 28813124 No abstract available.
-
Novel Treatments for Airway Disease.N Engl J Med. 2017 Aug 10;377(6):595-6. doi: 10.1056/NEJMc1708004. N Engl J Med. 2017. PMID: 28813132 No abstract available.
-
Novel Treatments for Airway Disease.N Engl J Med. 2017 Aug 10;377(6):596-7. doi: 10.1056/NEJMc1708004. N Engl J Med. 2017. PMID: 28813133 No abstract available.
-
Novel Treatments for Airway Disease.N Engl J Med. 2017 Aug 10;377(6):597. doi: 10.1056/NEJMc1708004. N Engl J Med. 2017. PMID: 28813135 No abstract available.
Similar articles
-
Benralizumab versus Mepolizumab for Eosinophilic Granulomatosis with Polyangiitis.N Engl J Med. 2024 Mar 7;390(10):911-921. doi: 10.1056/NEJMoa2311155. Epub 2024 Feb 23. N Engl J Med. 2024. PMID: 38393328 Clinical Trial.
-
Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis.J Allergy Clin Immunol. 2019 Jun;143(6):2170-2177. doi: 10.1016/j.jaci.2018.11.041. Epub 2018 Dec 19. J Allergy Clin Immunol. 2019. PMID: 30578883 Free PMC article. Clinical Trial.
-
Anti-cytokine targeted therapies for ANCA-associated vasculitis.Cochrane Database Syst Rev. 2020 Sep 29;9(9):CD008333. doi: 10.1002/14651858.CD008333.pub2. Cochrane Database Syst Rev. 2020. PMID: 32990324 Free PMC article.
-
Mepolizumab: 240563, anti-IL-5 monoclonal antibody - GlaxoSmithKline, anti-interleukin-5 monoclonal antibody - GlaxoSmithKline, SB 240563.Drugs R D. 2008;9(2):125-30. doi: 10.2165/00126839-200809020-00006. Drugs R D. 2008. PMID: 18298130 Review.
-
Treatment of Eosinophilic Granulomatosis with Polyangiitis: A Review.Drugs. 2018 Jun;78(8):809-821. doi: 10.1007/s40265-018-0920-8. Drugs. 2018. PMID: 29766394 Review.
Cited by
-
Eosinophils as Major Player in Type 2 Inflammation: Autoimmunity and Beyond.Adv Exp Med Biol. 2021;1347:197-219. doi: 10.1007/5584_2021_640. Adv Exp Med Biol. 2021. PMID: 34031864 Review.
-
Eosinophil Knockout Humans: Uncovering the Role of Eosinophils Through Eosinophil-Directed Biological Therapies.Annu Rev Immunol. 2021 Apr 26;39:719-757. doi: 10.1146/annurev-immunol-093019-125918. Epub 2021 Mar 1. Annu Rev Immunol. 2021. PMID: 33646859 Free PMC article.
-
Successful Treatment of Refractory Asthma Clinically Diagnosed As Eosinophilic Granulomatosis With Polyangiitis in a Hairdresser Using Tezepelumab.Cureus. 2024 Sep 3;16(9):e68570. doi: 10.7759/cureus.68570. eCollection 2024 Sep. Cureus. 2024. PMID: 39364473 Free PMC article.
-
A role for IL-33-activated ILC2s in eosinophilic vasculitis.JCI Insight. 2021 Jun 22;6(12):e143366. doi: 10.1172/jci.insight.143366. JCI Insight. 2021. PMID: 33974563 Free PMC article.
-
Looking Beyond Pneumonia and Asthma in India: An Interesting Case of Churg-Strauss Syndrome.Cureus. 2024 Aug 8;16(8):e66416. doi: 10.7759/cureus.66416. eCollection 2024 Aug. Cureus. 2024. PMID: 39246977 Free PMC article.
References
-
- Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome: clinical study and long-term follow-up of 96 patients. Medicine(Baltimore) 1999;78:26–37. - PubMed
-
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. - PubMed
-
- Cottin V, Bel E, Bottero P, et al. Respiratory manifestations of eosinophilic granulomatosis with polyangiitis (Churg-Strauss) Eur Respir J. 2016;48:1429–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
