Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development
- PMID: 28506998
- PMCID: PMC5482990
- DOI: 10.1242/dev.146019
Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development
Abstract
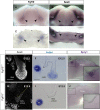
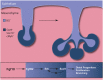
Salivary glands are formed by branching morphogenesis with epithelial progenitors forming a network of ducts and acini (secretory cells). During this process, epithelial progenitors specialise into distal (tips of the gland) and proximal (the stalk region) identities that produce the acini and higher order ducts, respectively. Little is known about the factors that regulate progenitor expansion and specialisation in the different parts of the gland. Here, we show that Sox9 is involved in establishing the identity of the distal compartment before the initiation of branching morphogenesis. Sox9 is expressed throughout the gland at the initiation stage before becoming restricted to the distal epithelium from the bud stage and throughout branching morphogenesis. Deletion of Sox9 in the epithelium results in loss of the distal epithelial progenitors, a reduction in proliferation and a subsequent failure in branching. We demonstrate that Sox9 is positively regulated by mesenchymal Fgf10, a process that requires active Erk signalling. These results provide new insights into the factors required for the expansion of salivary gland epithelial progenitors, which can be useful for organ regeneration therapy.
Keywords: Branching morphogenesis; Epithelial progenitors; Fgf signalling; Salivary glands; Sox9.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




Similar articles
-
Comparing development and regeneration in the submandibular gland highlights distinct mechanisms.J Anat. 2021 Jun;238(6):1371-1385. doi: 10.1111/joa.13387. Epub 2021 Jan 16. J Anat. 2021. PMID: 33455001 Free PMC article.
-
A branching morphogenesis program governs embryonic growth of the thyroid gland.Development. 2018 Jan 25;145(2):dev146829. doi: 10.1242/dev.146829. Development. 2018. PMID: 29361553 Free PMC article.
-
FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis.Development. 2005 Mar;132(6):1223-34. doi: 10.1242/dev.01690. Epub 2005 Feb 16. Development. 2005. PMID: 15716343
-
Regulatory Mechanisms Driving Salivary Gland Organogenesis.Curr Top Dev Biol. 2015;115:111-30. doi: 10.1016/bs.ctdb.2015.07.029. Epub 2015 Oct 21. Curr Top Dev Biol. 2015. PMID: 26589923 Free PMC article. Review.
-
Sox9 function in salivary gland development.J Oral Biosci. 2021 Mar;63(1):8-13. doi: 10.1016/j.job.2021.01.005. Epub 2021 Jan 23. J Oral Biosci. 2021. PMID: 33497841 Review.
Cited by
-
Epithelial Cell Lineage and Signaling in Murine Salivary Glands.J Dent Res. 2019 Oct;98(11):1186-1194. doi: 10.1177/0022034519864592. Epub 2019 Jul 22. J Dent Res. 2019. PMID: 31331226 Free PMC article. Review.
-
Sox10 Regulates Plasticity of Epithelial Progenitors toward Secretory Units of Exocrine Glands.Stem Cell Reports. 2019 Feb 12;12(2):366-380. doi: 10.1016/j.stemcr.2019.01.002. Epub 2019 Jan 31. Stem Cell Reports. 2019. PMID: 30713042 Free PMC article.
-
A novel role for cilia-dependent sonic hedgehog signaling during submandibular gland development.Dev Dyn. 2018 Jun;247(6):818-831. doi: 10.1002/dvdy.24627. Epub 2018 Apr 10. Dev Dyn. 2018. PMID: 29532549 Free PMC article.
-
Cell signaling regulation in salivary gland development.Cell Mol Life Sci. 2021 Apr;78(7):3299-3315. doi: 10.1007/s00018-020-03741-2. Epub 2021 Jan 15. Cell Mol Life Sci. 2021. PMID: 33449148 Free PMC article. Review.
-
Sox9 links biliary maturation to branching morphogenesis.bioRxiv [Preprint]. 2024 Jan 16:2024.01.15.574730. doi: 10.1101/2024.01.15.574730. bioRxiv. 2024. Update in: Nat Commun. 2025 Feb 15;16(1):1667. doi: 10.1038/s41467-025-56813-x. PMID: 38293117 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

