NFAM1 signaling enhances osteoclast formation and bone resorption activity in Paget's disease of bone
- PMID: 28506889
- PMCID: PMC5585872
- DOI: 10.1016/j.bone.2017.05.013
NFAM1 signaling enhances osteoclast formation and bone resorption activity in Paget's disease of bone
Abstract
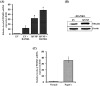
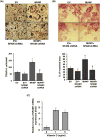
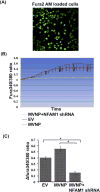
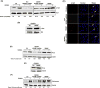
Paget's disease of bone (PDB) is marked by the focal activity of abnormal osteoclasts (OCLs) with excess bone resorption. We previously detected measles virus nucleocapsid protein (MVNP) transcripts in OCLs from patients with PDB. Also, MVNP stimulates pagetic OCL formation in vitro and in vivo. However, the mechanism by which MVNP induces excess OCLs/bone resorption activity in PDB is unclear. Microarray analysis identified MVNP induction of NFAM1 (NFAT activating protein with ITAM motif 1) expression. Therefore, we hypothesize that MVNP induction of NFAM1 enhances OCL differentiation and bone resorption in PDB. MVNP transduced normal human PBMC showed an increased NFAM1 mRNA expression without RANKL treatment. Further, bone marrow cells from patients with PDB demonstrated elevated levels of NFAM1 mRNA expression. Interestingly, shRNA suppression of NFAM1 inhibits MVNP induced OCL differentiation and bone resorption activity in mouse bone marrow cultures. Live cell widefield fluorescence microscopy analysis revealed that MVNP induced intracellular Ca2+ oscillations and levels were significantly reduced in NFAM1 suppressed preosteoclasts. Further, western blot analysis demonstrates that shRNA against NFAM1 inhibits MVNP stimulated PLCγ, calcineurin, and Syk activation in preosteoclast cells. Furthermore, NFAM1 expression controls NFATc1, a critical transcription factor expression and nuclear translocation in MVNP transuded preosteoclast cells. Thus, our results suggest that MVNP modulation of the NFAM1 signaling axis plays an essential role in pagetic OCL formation and bone resorption activity.
Keywords: Bone resorption; MVNP; NFAM1; Osteoclast; Paget's disease of bone.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Osteoclast inhibitory peptide-1 (OIP-1) inhibits measles virus nucleocapsid protein stimulated osteoclast formation/activity.J Cell Biochem. 2008 Jul 1;104(4):1500-8. doi: 10.1002/jcb.21723. J Cell Biochem. 2008. PMID: 18348201 Free PMC article.
-
Role of TAFII-17, a VDR binding protein, in the increased osteoclast formation in Paget's Disease.J Bone Miner Res. 2004 Jul;19(7):1154-64. doi: 10.1359/JBMR.040312. Epub 2004 Mar 15. J Bone Miner Res. 2004. PMID: 15176999
-
Expression of measles virus nucleocapsid protein in osteoclasts induces Paget's disease-like bone lesions in mice.J Bone Miner Res. 2006 Mar;21(3):446-55. doi: 10.1359/JBMR.051108. Epub 2005 Nov 21. J Bone Miner Res. 2006. PMID: 16491293
-
Experimental models of Paget's disease.J Bone Miner Res. 2006 Dec;21 Suppl 2:P55-7. doi: 10.1359/jbmr.06s210. J Bone Miner Res. 2006. PMID: 17229020 Review.
-
Insights into the pathogenesis of Paget's disease.Ann N Y Acad Sci. 2010 Mar;1192:176-80. doi: 10.1111/j.1749-6632.2009.05214.x. Ann N Y Acad Sci. 2010. PMID: 20392234 Review.
Cited by
-
Inhibition of NFAM1 suppresses phospho-SAPK/JNK signaling during osteoclast differentiation and bone resorption.J Cell Biochem. 2021 Oct;122(10):1534-1543. doi: 10.1002/jcb.30076. Epub 2021 Jul 6. J Cell Biochem. 2021. PMID: 34228377 Free PMC article.
-
NFAM1 Promotes Pro-Inflammatory Cytokine Production in Mouse and Human Monocytes.Front Immunol. 2022 Jan 13;12:773445. doi: 10.3389/fimmu.2021.773445. eCollection 2021. Front Immunol. 2022. PMID: 35095847 Free PMC article.
-
Autoregulation of RANK ligand in oral squamous cell carcinoma tumor cells.J Cell Physiol. 2018 Aug;233(8):6125-6134. doi: 10.1002/jcp.26456. Epub 2018 Mar 6. J Cell Physiol. 2018. PMID: 29323724 Free PMC article.
-
Whole-transcriptome sequencing of knee joint cartilage from osteoarthritis patients.Bone Joint Res. 2019 Aug 2;8(7):290-303. doi: 10.1302/2046-3758.87.BJR-2018-0297.R1. eCollection 2019 Jul. Bone Joint Res. 2019. PMID: 31463037 Free PMC article.
-
METTL3 induces bone marrow mesenchymal stem cells osteogenic differentiation and migration through facilitating M1 macrophage differentiation.Am J Transl Res. 2021 May 15;13(5):4376-4388. eCollection 2021. Am J Transl Res. 2021. PMID: 34150020 Free PMC article.
References
-
- Vallet M, Ralston SH. Biology and treatment of Paget's disease of bone. J. Cell. Biochem. 2016;117(2):289–299. - PubMed
-
- Hamdy RC. Clinical features and pharmacologic treatment of Paget's disease. Endocrinol. Metab. Clin. N. Am. 1995;24(2):421–436. - PubMed
-
- Aoki M, Tanahashi S, Mizuta K, Kato H. Treatment for progressive hearing loss due to Paget's disease of bone - a case report and literature review. J. Int. Adv. Otol. 2015;11(3):267–270. - PubMed
-
- Leach RJ, Singer FR, Roodman GD. The genetics of Paget's disease of the bone. J. Clin. Endocrinol. Metab. 2001;86(1):24–28. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

