Cost-Effectiveness of Dengue Vaccination Programs in Brazil
- PMID: 28500811
- PMCID: PMC5417221
- DOI: 10.4269/ajtmh.16-0810
Cost-Effectiveness of Dengue Vaccination Programs in Brazil
Abstract
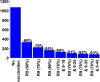
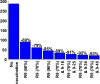
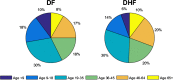
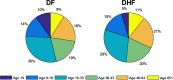
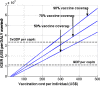
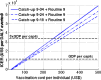
AbstractThe first approved dengue vaccine, CYD-TDV, a chimeric, live-attenuated, tetravalent dengue virus vaccine, was recently licensed in 13 countries, including Brazil. In light of recent vaccine approval, we modeled the cost-effectiveness of potential vaccination policies mathematically based on data from recent vaccine efficacy trials that indicated that vaccine efficacy was lower in seronegative individuals than in seropositive individuals. In our analysis, we investigated several vaccination programs, including routine vaccination, with various vaccine coverage levels and those with and without large catch-up campaigns. As it is unclear whether the vaccine protects against infection or just against disease, our model incorporated both direct and indirect effects of vaccination. We found that in the presence of vaccine-induced indirect protection, the cost-effectiveness of dengue vaccination decreased with increasing vaccine coverage levels because the marginal returns of herd immunity decreases with vaccine coverage. All routine dengue vaccination programs that we considered were cost-effective, reducing dengue incidence significantly. Specifically, a routine dengue vaccination of 9-year-olds would be cost-effective when the cost of vaccination per individual is less than $262. Furthermore, the combination of routine vaccination and large catch-up campaigns resulted in a greater reduction of dengue burden (by up to 93%) than routine vaccination alone, making it a cost-effective intervention as long as the cost per course of vaccination is $255 or less. Our results show that dengue vaccination would be cost-effective in Brazil even with a relatively low vaccine efficacy in seronegative individuals.
Figures
Similar articles
-
A multi-country study of dengue vaccination strategies with Dengvaxia and a future vaccine candidate in three dengue-endemic countries: Vietnam, Thailand, and Colombia.Vaccine. 2018 Apr 19;36(17):2346-2355. doi: 10.1016/j.vaccine.2018.03.002. Epub 2018 Mar 21. Vaccine. 2018. PMID: 29573874
-
Dengue dynamics and vaccine cost-effectiveness in Brazil.Vaccine. 2013 Aug 20;31(37):3957-61. doi: 10.1016/j.vaccine.2013.06.036. Epub 2013 Jun 20. Vaccine. 2013. PMID: 23791696 Free PMC article.
-
Assessment of bivalent and tetravalent dengue vaccine formulations in flavivirus-naïve adults in Mexico.Hum Vaccin Immunother. 2014;10(10):2853-63. doi: 10.4161/21645515.2014.972131. Hum Vaccin Immunother. 2014. PMID: 25483647 Free PMC article. Clinical Trial.
-
[Analysis of the evidence on the efficacy and safety of CYD-TDV dengue vaccine and its potential licensing and implementation through Mexico's Universal Vaccination Program].Salud Publica Mex. 2016 Jan-Feb;58(1):71-83. Salud Publica Mex. 2016. PMID: 26879510 Review. Spanish.
-
Current issues in the economics of vaccination against dengue.Expert Rev Vaccines. 2016;15(4):519-28. doi: 10.1586/14760584.2016.1129278. Epub 2016 Jan 4. Expert Rev Vaccines. 2016. PMID: 26642099 Review.
Cited by
-
Model-based assessment of public health impact and cost-effectiveness of dengue vaccination following screening for prior exposure.PLoS Negl Trop Dis. 2019 Jul 1;13(7):e0007482. doi: 10.1371/journal.pntd.0007482. eCollection 2019 Jul. PLoS Negl Trop Dis. 2019. PMID: 31260441 Free PMC article.
-
"To do, or not to do?": determinants of stakeholders' acceptance on dengue vaccine using PLS-SEM analysis in Malaysia.BMC Public Health. 2022 Aug 19;22(1):1574. doi: 10.1186/s12889-022-13967-3. BMC Public Health. 2022. PMID: 35982443 Free PMC article.
-
Inclusion of Additional Unintended Consequences in Economic Evaluation: A Systematic Review of Immunization and Tuberculosis Cost-Effectiveness Analyses.Pharmacoecon Open. 2021 Dec;5(4):587-603. doi: 10.1007/s41669-021-00269-4. Epub 2021 May 4. Pharmacoecon Open. 2021. PMID: 33948928 Free PMC article.
-
Comparative evaluation of an improved test method for bioefficacy of insecticidal fabrics against dengue and malaria vectors.Parasit Vectors. 2019 Jul 29;12(1):375. doi: 10.1186/s13071-019-3637-y. Parasit Vectors. 2019. PMID: 31358045 Free PMC article.
-
A review on test methods for insecticidal fabrics and the need for standardisation.Parasitol Res. 2018 Oct;117(10):3067-3080. doi: 10.1007/s00436-018-6061-x. Epub 2018 Aug 27. Parasitol Res. 2018. PMID: 30151634 Review.
References
-
- World Health Organization . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva, Switzerland: World Health Organization; 2009. - PubMed
-
- Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. - PubMed
-
- Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

