Effect of macrophages on breast cancer cell proliferation, and on expression of hormone receptors, uPAR and HER-2
- PMID: 28498427
- PMCID: PMC5467790
- DOI: 10.3892/ijo.2017.3996
Effect of macrophages on breast cancer cell proliferation, and on expression of hormone receptors, uPAR and HER-2
Abstract
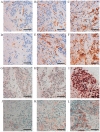
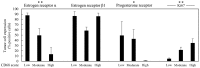
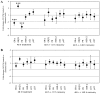
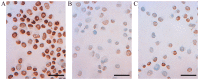
Malignant tumors, including breast cancers, are frequently infiltrated with innate immune cells and tumor-associated macrophages (TAMs) represent the major inflammatory component in stroma of many tumors. In this study, we examined the immunoreactivity of the macrophage markers CD68 and CD163 as well as the hormone receptors estrogen receptor α (ERα), progesterone receptor (PR), estrogen receptor β1 (ERβ1), human epidermal growth factor receptor 2 (HER-2), matrix metalloproteinase 9 (MMP‑9), urokinase-type plasminogen activator receptor (uPAR) and the proliferations marker Ki67 in 17 breast cancer biopsies. The quantitative score for CD68+ and CD163+ strongly indicate M2 phenotype dominance in the currently investigated biopsies. We found that an increasing level of macrophages was negatively associated with ERα or PR, whereas a positive association was observed for Ki-67 or uPAR. No significant association could be seen between the level of macrophage and HER-2, ERβ1 or MMP-9 expression. Effect of conditioned media (CM) generated from cultured human M1 and M2 macrophage phenotypes were investigated on the proliferation and expression of selected markers in the T47D breast cancer cell line. We found that in contrast to the in vivo situation, in particularly the CM from M1 macrophages decreased the growth and Ki67 expression in T47D, and significantly increased ERβ1 mRNA levels. Moreover, in accordance to the in vivo situation the CM from the macrophages decreased the expression of ERα protein as well as ERα or PR mRNA. In conclusion our results show that macrophages alone have the capability to decrease the tumor cell expression of ERα and PR in vitro. In the tumor environment in vivo macrophages also contribute to an increase in tumor cell expression of uPAR and Ki67, suggesting that macrophages are involved in impairing the prognosis for breast cancer patients.
Figures


Similar articles
-
Macrophages of M1 phenotype have properties that influence lung cancer cell progression.Tumour Biol. 2015 Nov;36(11):8715-25. doi: 10.1007/s13277-015-3630-9. Epub 2015 Jun 7. Tumour Biol. 2015. PMID: 26050228
-
Human breast cancer cells educate macrophages toward the M2 activation status.Breast Cancer Res. 2015 Aug 5;17(1):101. doi: 10.1186/s13058-015-0621-0. Breast Cancer Res. 2015. PMID: 26243145 Free PMC article.
-
CD68, CD163, and matrix metalloproteinase 9 (MMP-9) co-localization in breast tumor microenvironment predicts survival differently in ER-positive and -negative cancers.Breast Cancer Res. 2018 Dec 17;20(1):154. doi: 10.1186/s13058-018-1076-x. Breast Cancer Res. 2018. PMID: 30558648 Free PMC article.
-
Co-expression of steroid receptors (estrogen receptor alpha and/or progesterone receptors) and Her-2/neu: Clinical implications.J Steroid Biochem Mol Biol. 2006 Dec;102(1-5):32-40. doi: 10.1016/j.jsbmb.2006.09.008. Epub 2006 Oct 17. J Steroid Biochem Mol Biol. 2006. PMID: 17049840 Review.
-
Relationships between progesterone receptor isoforms and the HER/ErbB receptors and ligands network in 299 primary breast cancers.Int J Biol Markers. 2012 Jul 19;27(2):e111-7. doi: 10.5301/JBM.2012.9198. Int J Biol Markers. 2012. PMID: 22505232 Review.
Cited by
-
Comprehensive analysis of the importance of PLAUR in the progression and immune microenvironment of renal clear cell carcinoma.PLoS One. 2022 Jun 8;17(6):e0269595. doi: 10.1371/journal.pone.0269595. eCollection 2022. PLoS One. 2022. PMID: 35675366 Free PMC article.
-
Macrophage subtypes inhibit breast cancer proliferation in culture.bioRxiv [Preprint]. 2024 Jun 1:2024.06.01.596963. doi: 10.1101/2024.06.01.596963. bioRxiv. 2024. Update in: Mol Biol Cell. 2025 Jan 1;36(1):br2. doi: 10.1091/mbc.E24-06-0241. PMID: 38853881 Free PMC article. Updated. Preprint.
-
RNA-seq and ATAC-seq analysis of CD163+ macrophage-induced progestin-insensitive endometrial cancer cells.Cancer Med. 2023 Mar;12(5):5964-5978. doi: 10.1002/cam4.5396. Epub 2022 Nov 14. Cancer Med. 2023. PMID: 36373483 Free PMC article.
-
MicroRNA-200c restoration reveals a cytokine profile to enhance M1 macrophage polarization in breast cancer.NPJ Breast Cancer. 2021 May 27;7(1):64. doi: 10.1038/s41523-021-00273-1. NPJ Breast Cancer. 2021. PMID: 34045467 Free PMC article.
-
Evaluation of Number Density of Tumor-Associated Macrophages by Immunohistochemistry and Semiquantitative Scoring in Invasive Breast Cancer: An Indian Study.J Microsc Ultrastruct. 2022 Sep 6;11(4):214-219. doi: 10.4103/jmau.jmau_51_21. eCollection 2023 Oct-Dec. J Microsc Ultrastruct. 2022. PMID: 38213652 Free PMC article.
References
-
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Panel members: Strategies for subtypes - dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

