HIV-1 TAT protein enhances sensitization to methamphetamine by affecting dopaminergic function
- PMID: 28495611
- PMCID: PMC5537017
- DOI: 10.1016/j.bbi.2017.05.004
HIV-1 TAT protein enhances sensitization to methamphetamine by affecting dopaminergic function
Abstract
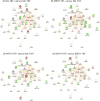
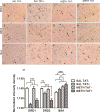
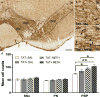
Methamphetamine abuse is common among humans with immunodeficiency virus (HIV). The HIV-1 regulatory protein TAT induces dysfunction of mesolimbic dopaminergic systems which may result in impaired reward processes and contribute to methamphetamine abuse. These studies investigated the impact of TAT expression on methamphetamine-induced locomotor sensitization, underlying changes in dopamine function and adenosine receptors in mesolimbic brain areas and neuroinflammation (microgliosis). Transgenic mice with doxycycline-induced TAT protein expression in the brain were tested for locomotor activity in response to repeated methamphetamine injections and methamphetamine challenge after a 7-day abstinence period. Dopamine function in the nucleus accumbens (Acb) was determined using high performance liquid chromatography. Expression of dopamine and/or adenosine A receptors (ADORA) in the Acb and caudate putamen (CPu) was assessed using RT-PCR and immunohistochemistry analyses. Microarrays with pathway analyses assessed dopamine and adenosine signaling in the CPu. Activity-dependent neurotransmitter switching of a reserve pool of non-dopaminergic neurons to a dopaminergic phenotype in the ventral tegmental area (VTA) was determined by immunohistochemistry and quantified with stereology. TAT expression enhanced methamphetamine-induced sensitization. TAT expression alone decreased striatal dopamine (D1, D2, D4, D5) and ADORA1A receptor expression, while increasing ADORA2A receptors expression. Moreover, TAT expression combined with methamphetamine exposure was associated with increased adenosine A receptors (ADORA1A) expression and increased recruitment of dopamine neurons in the VTA. TAT expression and methamphetamine exposure induced microglia activation with the largest effect after combined exposure. Our findings suggest that dopamine-adenosine receptor interactions and reserve pool neuronal recruitment may represent potential targets to develop new treatments for methamphetamine abuse in individuals with HIV.
Keywords: Adenosine receptors; Brain neurochemistry; Dopamine receptors; Gene expression microarrays; HPLC; Locomotor activity; Mice; Neurotransmitter respecification; TAT expression.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
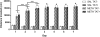
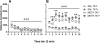
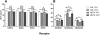
Similar articles
-
Sex differences and Tat expression affect dopaminergic receptor expression and response to antioxidant treatment in methamphetamine-sensitized HIV Tat transgenic mice.Neuropharmacology. 2020 Nov 1;178:108245. doi: 10.1016/j.neuropharm.2020.108245. Epub 2020 Aug 9. Neuropharmacology. 2020. PMID: 32783894 Free PMC article.
-
Brain Reward Function after Chronic and Binge Methamphetamine Regimens in Mice Expressing the HIV-1 TAT Protein.Curr HIV Res. 2019;17(2):126-133. doi: 10.2174/1570162X17666190703165408. Curr HIV Res. 2019. PMID: 31269883 Free PMC article.
-
The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice.Neuropharmacology. 2016 Oct;109:205-215. doi: 10.1016/j.neuropharm.2016.06.011. Epub 2016 Jun 15. Neuropharmacology. 2016. PMID: 27316905 Free PMC article.
-
Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions.Ann N Y Acad Sci. 2001 Jun;937:93-120. doi: 10.1111/j.1749-6632.2001.tb03560.x. Ann N Y Acad Sci. 2001. PMID: 11458542 Review.
-
[Purinergic modulation of the brain dopaminergic transmission: behavioral-pharmacologic conclusions].Neuropsychopharmacol Hung. 2008 May;10(2):97-102. Neuropsychopharmacol Hung. 2008. PMID: 18959141 Review. Hungarian.
Cited by
-
Both HIV and Tat expression decrease prepulse inhibition with further impairment by methamphetamine.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Mar 2;106:110089. doi: 10.1016/j.pnpbp.2020.110089. Epub 2020 Sep 4. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 32891668 Free PMC article.
-
Detection of H3K4me3 Identifies NeuroHIV Signatures, Genomic Effects of Methamphetamine and Addiction Pathways in Postmortem HIV+ Brain Specimens that Are Not Amenable to Transcriptome Analysis.Viruses. 2021 Mar 24;13(4):544. doi: 10.3390/v13040544. Viruses. 2021. PMID: 33805201 Free PMC article.
-
Identifying methamphetamine use predictors in HIV infection: Immune-dopaminergic signatures in peripheral leukocytes and the role of COMT genotype.Brain Behav Immun Health. 2024 Oct 5;42:100873. doi: 10.1016/j.bbih.2024.100873. eCollection 2024 Dec. Brain Behav Immun Health. 2024. PMID: 39430881 Free PMC article.
-
Methamphetamine and Cannabis: A Tale of Two Drugs and their Effects on HIV, Brain, and Behavior.J Neuroimmune Pharmacol. 2020 Dec;15(4):743-764. doi: 10.1007/s11481-020-09957-0. Epub 2020 Sep 15. J Neuroimmune Pharmacol. 2020. PMID: 32929575 Free PMC article. Review.
-
Sex differences and Tat expression affect dopaminergic receptor expression and response to antioxidant treatment in methamphetamine-sensitized HIV Tat transgenic mice.Neuropharmacology. 2020 Nov 1;178:108245. doi: 10.1016/j.neuropharm.2020.108245. Epub 2020 Aug 9. Neuropharmacology. 2020. PMID: 32783894 Free PMC article.
References
-
- Boekhoudt L, Omrani A, Luijendijk MC, Wolterink-Donselaar IG, Wijbrans EC, van der Plasse G, Adan RA. Chemogenetic activation of dopamine neurons in the ventral tegmental area, but not substantia nigra, induces hyperactivity in rats. Eur Neuropsychopharmacol. 2016;26:1784–1793. - PubMed
-
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nature Neuroscience. 2014;17:1022–1030. - PubMed
-
- Chesworth R, Brown RM, Kim JH, Ledent C, Lawrence AJ. Adenosine 2A receptors modulate reward behaviours for methamphetamine. Addict Biol. 2016;21:407–421. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

