IDO, PTEN-expressing Tregs and control of antigen-presentation in the murine tumor microenvironment
- PMID: 28488123
- PMCID: PMC5627605
- DOI: 10.1007/s00262-017-2010-2
IDO, PTEN-expressing Tregs and control of antigen-presentation in the murine tumor microenvironment
Abstract
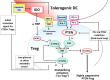
The tumor microenvironment is profoundly immunosuppressive. This creates a major barrier for attempts to combine immunotherapy with conventional chemotherapy or radiation, because the tumor antigens released by these cytotoxic agents are not cross-presented in an immunogenic fashion. In this Focused Research Review, we focus on mouse preclinical studies exploring the role of immunosuppressive Tregs expressing the PTEN lipid phosphatase, and the links between PTEN+ Tregs and the immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO). IDO has received attention because it can be expressed by a variety of human tumor types in vivo, but IDO can also be induced in host immune cells of both humans and mice in response to inflammation, infection or dying (apoptotic) cells. Mechanistically, IDO and PTEN+ Tregs are closely connected, with IDO causing activation of the PTEN pathway in Tregs. Genetic ablation or pharmacologic inhibition of PTEN in mouse Tregs destabilizes their suppressive phenotype, and this prevents transplantable and autochthonous tumors from creating their normal immunosuppressive microenvironment. Genetic ablation of either IDO or PTEN+ Tregs in mice results in a fundamental defect in the ability to maintain tolerance to antigens associated with apoptotic cells, including dying tumor cells. Consistent with this, pharmacologic inhibitors of either pathway show synergy when combined with cytotoxic agents such as chemotherapy or radiation. Thus, we propose that IDO and PTEN+ Tregs represent closely linked checkpoints that can influence the choice between immune activation versus tolerance to dying tumor cells.
Keywords: Chemotherapy; Indoleamine 2,3-dioxygenase; PTEN; Regulatory T cells; Regulatory myeloid suppressor cells; Tolerance.
Conflict of interest statement
David Munn is a consultant to NewLink Genetics Corporation and holds intellectual property in IDO-inhibitors and PTEN-inhibitors. Theodore Johnson receives funding for clinical trials of IDO-inhibitors from NewLink Genetics, Inc. The authors declare that there are no other conflicts of interest.
Figures
Similar articles
-
Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase.J Clin Invest. 2007 Sep;117(9):2570-82. doi: 10.1172/JCI31911. J Clin Invest. 2007. PMID: 17710230 Free PMC article.
-
Neem leaf glycoprotein overcomes indoleamine 2,3 dioxygenase mediated tolerance in dendritic cells by attenuating hyperactive regulatory T cells in cervical cancer stage IIIB patients.Hum Immunol. 2013 Aug;74(8):1015-23. doi: 10.1016/j.humimm.2013.04.022. Epub 2013 Apr 27. Hum Immunol. 2013. PMID: 23628394
-
The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment.Sci Adv. 2015 Nov 6;1(10):e1500845. doi: 10.1126/sciadv.1500845. eCollection 2015 Nov. Sci Adv. 2015. PMID: 26601142 Free PMC article.
-
IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance.Trends Immunol. 2016 Mar;37(3):193-207. doi: 10.1016/j.it.2016.01.002. Epub 2016 Jan 31. Trends Immunol. 2016. PMID: 26839260 Free PMC article. Review.
-
Chemo-Immunotherapy: Role of Indoleamine 2,3-Dioxygenase in Defining Immunogenic Versus Tolerogenic Cell Death in the Tumor Microenvironment.Adv Exp Med Biol. 2017;1036:91-104. doi: 10.1007/978-3-319-67577-0_7. Adv Exp Med Biol. 2017. PMID: 29275467 Free PMC article. Review.
Cited by
-
Synergistic Effects of Cabozantinib and EGFR-Specific CAR-NK-92 Cells in Renal Cell Carcinoma.J Immunol Res. 2017;2017:6915912. doi: 10.1155/2017/6915912. Epub 2017 Dec 20. J Immunol Res. 2017. PMID: 29423418 Free PMC article.
-
Metabolic Stress and Immunity: Nutrient-Sensing Kinases and Tryptophan Metabolism.Adv Exp Med Biol. 2021;1275:395-405. doi: 10.1007/978-3-030-49844-3_16. Adv Exp Med Biol. 2021. PMID: 33539025
-
Molecular mechanisms and therapeutic significance of Tryptophan Metabolism and signaling in cancer.Mol Cancer. 2024 Oct 30;23(1):241. doi: 10.1186/s12943-024-02164-y. Mol Cancer. 2024. PMID: 39472902 Free PMC article. Review.
-
PTEN Function at the Interface between Cancer and Tumor Microenvironment: Implications for Response to Immunotherapy.Int J Mol Sci. 2020 Jul 27;21(15):5337. doi: 10.3390/ijms21155337. Int J Mol Sci. 2020. PMID: 32727102 Free PMC article. Review.
-
Inflammatory Reprogramming with IDO1 Inhibitors: Turning Immunologically Unresponsive 'Cold' Tumors 'Hot'.Trends Cancer. 2018 Jan;4(1):38-58. doi: 10.1016/j.trecan.2017.11.005. Epub 2017 Dec 21. Trends Cancer. 2018. PMID: 29413421 Free PMC article. Review.
References
-
- McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. - DOI - PMC - PubMed
-
- Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJ, van der Burg S, Kapiteijn E, Michielin O, Romano E, Linnemann C, Speiser D, Blank C, Haanen JB, Schumacher TN. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128. doi: 10.1126/scitranslmed.3008918. - DOI - PubMed
-
- Reissfelder C, Stamova S, Gossmann C, Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M, Saadati M, Benner A, Buchler MW, Jager D, Halama N, Khazaie K, Weitz J, Beckhove P. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125:739–751. doi: 10.1172/JCI74894. - DOI - PMC - PubMed
-
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials