Immune Modulatory Cell Therapy for Hemophilia B Based on CD20-Targeted Lentiviral Gene Transfer to Primary B Cells
- PMID: 28480307
- PMCID: PMC5415320
- DOI: 10.1016/j.omtm.2017.03.005
Immune Modulatory Cell Therapy for Hemophilia B Based on CD20-Targeted Lentiviral Gene Transfer to Primary B Cells
Abstract
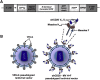
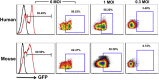
Gene-modified B cells expressing immunoglobulin G (IgG) fusion proteins have been shown to induce tolerance in several autoimmune and other disease models. However, lack of a vector suitable for gene transfer to human B cells has been an obstacle for translation of this approach. To overcome this hurdle, we developed an IgG-human factor IX (hFIX) lentiviral fusion construct that was targeted to specifically transduce cells expressing human CD20 (hCD20). Receptor-specific retargeting by mutating envelope glycoproteins of measles virus (MV)-lentiviral vector (LV) and addition of a single-chain variable fragment specific for hCD20 resulted in gene delivery into primary human and transgenic hCD20 mouse B cells with high specificity. Notably, this protocol neither required nor induced activation of the B cells, as confirmed by minimal activation of inflammatory cytokines. Using this strategy, we were able to demonstrate induction of humoral tolerance, resulting in suppression of antibody formation against hFIX in a mouse model of hemophilia B (HB). In conclusion, transduction of receptor-specific retargeted LV into resting B cells is a promising method to develop B cell therapies for antigen-specific tolerance induction in human disease.
Keywords: CD20; SCFV; gene transfer; hemophilia B; lentivirus; measles virus; psuedotype.
Figures



Similar articles
-
CD19 and CD20 targeted vectors induce minimal activation of resting B lymphocytes.PLoS One. 2013 Nov 11;8(11):e79047. doi: 10.1371/journal.pone.0079047. eCollection 2013. PLoS One. 2013. PMID: 24244415 Free PMC article.
-
Optimized Lentiviral Transduction Protocols by Use of a Poloxamer Enhancer, Spinoculation, and scFv-Antibody Fusions to VSV-G.Methods Mol Biol. 2016;1448:49-61. doi: 10.1007/978-1-4939-3753-0_4. Methods Mol Biol. 2016. PMID: 27317172
-
Systematic improvement of lentivirus transduction protocols by antibody fragments fused to VSV-G as envelope glycoprotein.Biomaterials. 2014 Apr;35(13):4204-12. doi: 10.1016/j.biomaterials.2014.01.051. Epub 2014 Feb 12. Biomaterials. 2014. PMID: 24529898
-
Theodore E. Woodward Award. AAV-mediated gene transfer for hemophilia.Trans Am Clin Climatol Assoc. 2003;114:337-51; discussion 351-2. Trans Am Clin Climatol Assoc. 2003. PMID: 12813929 Free PMC article. Review.
-
Hemophilia A gene therapy via intraosseous delivery of factor VIII-lentiviral vectors.Thromb J. 2016 Oct 4;14(Suppl 1):41. doi: 10.1186/s12959-016-0105-1. eCollection 2016. Thromb J. 2016. PMID: 27766066 Free PMC article. Review.
Cited by
-
Immune Responses to Viral Gene Therapy Vectors.Mol Ther. 2020 Mar 4;28(3):709-722. doi: 10.1016/j.ymthe.2020.01.001. Epub 2020 Jan 10. Mol Ther. 2020. PMID: 31968213 Free PMC article. Review.
-
Innovative Approaches for Immune Tolerance to Factor VIII in the Treatment of Hemophilia A.Front Immunol. 2017 Nov 24;8:1604. doi: 10.3389/fimmu.2017.01604. eCollection 2017. Front Immunol. 2017. PMID: 29225598 Free PMC article. Review.
-
Regulatory T cells and TLR9 activation shape antibody formation to a secreted transgene product in AAV muscle gene transfer.Cell Immunol. 2019 Aug;342:103682. doi: 10.1016/j.cellimm.2017.07.012. Epub 2017 Aug 1. Cell Immunol. 2019. PMID: 28888664 Free PMC article.
-
Tolerance induction in hemophilia: innovation and accomplishments.Curr Opin Hematol. 2018 Sep;25(5):365-372. doi: 10.1097/MOH.0000000000000446. Curr Opin Hematol. 2018. PMID: 29994897 Free PMC article. Review.
-
Surface-Engineered Lentiviral Vectors for Selective Gene Transfer into Subtypes of Lymphocytes.Mol Ther Methods Clin Dev. 2018 Oct 17;12:19-31. doi: 10.1016/j.omtm.2018.10.006. eCollection 2019 Mar 15. Mol Ther Methods Clin Dev. 2018. PMID: 30417026 Free PMC article. Review.
References
-
- DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br. J. Haematol. 2007;138:305–315. - PubMed
-
- DiMichele D.M. Immune tolerance in haemophilia: the long journey to the fork in the road. Br. J. Haematol. 2012;159:123–134. - PubMed
-
- Chitlur M., Warrier I., Rajpurkar M., Lusher J.M. Inhibitors in factor IX deficiency a report of the ISTH-SSC international FIX inhibitor registry (1997-2006) Haemophilia. 2009;15:1027–1031. - PubMed
-
- Warrier I. Blackwell Publishing; 2005. Inhibitors in Hemophilia B.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

