Detection of Emerging Vaccine-Related Polioviruses by Deep Sequencing
- PMID: 28468861
- PMCID: PMC5483918
- DOI: 10.1128/JCM.00144-17
Detection of Emerging Vaccine-Related Polioviruses by Deep Sequencing
Abstract
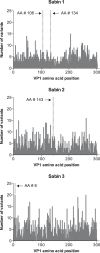
Oral poliovirus vaccine can mutate to regain neurovirulence. To date, evaluation of these mutations has been performed primarily on culture-enriched isolates by using conventional Sanger sequencing. We therefore developed a culture-independent, deep-sequencing method targeting the 5' untranslated region (UTR) and P1 genomic region to characterize vaccine-related poliovirus variants. Error analysis of the deep-sequencing method demonstrated reliable detection of poliovirus mutations at levels of <1%, depending on read depth. Sequencing of viral nucleic acids from the stool of vaccinated, asymptomatic children and their close contacts collected during a prospective cohort study in Veracruz, Mexico, revealed no vaccine-derived polioviruses. This was expected given that the longest duration between sequenced sample collection and the end of the most recent national immunization week was 66 days. However, we identified many low-level variants (<5%) distributed across the 5' UTR and P1 genomic region in all three Sabin serotypes, as well as vaccine-related viruses with multiple canonical mutations associated with phenotypic reversion present at high levels (>90%). These results suggest that monitoring emerging vaccine-related poliovirus variants by deep sequencing may aid in the poliovirus endgame and efforts to ensure global polio eradication.
Keywords: enterovirus; high-throughput nucleotide sequencing; molecular methods; oral; oral vaccines; poliovirus; poliovirus vaccine.
Copyright © 2017 Sahoo et al.
Figures

Similar articles
-
Deep sequencing prompts the modification of a real-time RT-PCR for the serotype-specific detection of polioviruses.J Virol Methods. 2019 Feb;264:38-43. doi: 10.1016/j.jviromet.2018.11.007. Epub 2018 Nov 14. J Virol Methods. 2019. PMID: 30447245 Free PMC article.
-
Prevalence of vaccine-derived polioviruses in stools of immunodeficient children in South Africa.J Appl Microbiol. 2006 Dec;101(6):1367-79. doi: 10.1111/j.1365-2672.2006.03020.x. J Appl Microbiol. 2006. PMID: 17105568
-
Sabin Vaccine Reversion in the Field: a Comprehensive Analysis of Sabin-Like Poliovirus Isolates in Nigeria.J Virol. 2015 Oct 14;90(1):317-31. doi: 10.1128/JVI.01532-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468545 Free PMC article.
-
Neurologic complications associated with oral poliovirus vaccine and genomic variability of the vaccine strains after multiplication in humans.Acta Virol. 1998 Jun;42(3):187-94. Acta Virol. 1998. PMID: 9842449 Review.
-
Risks associated with the use of live-attenuated vaccine poliovirus strains and the strategies for control and eradication of paralytic poliomyelitis.Expert Rev Vaccines. 2012 May;11(5):609-28. doi: 10.1586/erv.12.28. Expert Rev Vaccines. 2012. PMID: 22827246 Review.
Cited by
-
Multiplex SARS-CoV-2 Genotyping Reverse Transcriptase PCR for Population-Level Variant Screening and Epidemiologic Surveillance.J Clin Microbiol. 2021 Jul 19;59(8):e0085921. doi: 10.1128/JCM.00859-21. Epub 2021 Jul 19. J Clin Microbiol. 2021. PMID: 34037430 Free PMC article.
-
The Early Evolution of Oral Poliovirus Vaccine Is Shaped by Strong Positive Selection and Tight Transmission Bottlenecks.Cell Host Microbe. 2021 Jan 13;29(1):32-43.e4. doi: 10.1016/j.chom.2020.10.011. Epub 2020 Nov 18. Cell Host Microbe. 2021. PMID: 33212020 Free PMC article.
-
Attenuation of Live-Attenuated Yellow Fever 17D Vaccine Virus Is Localized to a High-Fidelity Replication Complex.mBio. 2019 Oct 22;10(5):e02294-19. doi: 10.1128/mBio.02294-19. mBio. 2019. PMID: 31641088 Free PMC article.
-
Deep sequencing prompts the modification of a real-time RT-PCR for the serotype-specific detection of polioviruses.J Virol Methods. 2019 Feb;264:38-43. doi: 10.1016/j.jviromet.2018.11.007. Epub 2018 Nov 14. J Virol Methods. 2019. PMID: 30447245 Free PMC article.
-
Protocol Paper: Oral Poliovirus Vaccine Transmissibility in Communities After Cessation of Routine Oral Poliovirus Vaccine Immunization.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S115-S120. doi: 10.1093/cid/ciy606. Clin Infect Dis. 2018. PMID: 30376084 Free PMC article. Clinical Trial.
References
-
- World Health Organization. 2003. Report of the interim meeting of the Technical Consultation Group (TCG) on the global eradication of poliomyelitis, Geneva, 13-14 November 2002. World Health Organization, Geneva, Switzerland.
-
- Plotkin SA, Orenstein WA, Offit PA. 2013. Vaccines, 6th ed Elsevier Saunders, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

