Dendritic cell-targeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae
- PMID: 28463465
- PMCID: PMC5529118
- DOI: 10.1111/1348-0421.12487
Dendritic cell-targeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae
Abstract
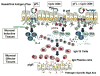
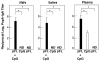
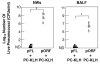
To develop safe vaccines for inducing mucosal immunity to major pulmonary bacterial infections, appropriate vaccine antigens (Ags), delivery systems and nontoxic molecular adjuvants must be considered. Such vaccine constructs can induce Ag-specific immune responses that protect against mucosal infections. In particular, it has been shown that simply mixing the adjuvant with the bacterial Ag is a relatively easy means of constructing adjuvant-based mucosal vaccine preparations; the resulting vaccines can elicit protective immunity. DNA-based nasal adjuvants targeting mucosal DCs have been studied in order to induce Ag-specific mucosal and systemic immune responses that provide essential protection against microbial pathogens that invade mucosal surfaces. In this review, initially a plasmid encoding the cDNA of Flt3 ligand (pFL), a molecule that is a growth factor for DCs, as an effective adjuvant for mucosal immunity to pneumococcal infections, is introduced. Next, the potential of adding unmethylated CpG oligodeoxynucleotide and pFL together with a pneumococcal Ag to induce protection from pneumococcal infections is discussed. Pneumococcal surface protein A has been used as vaccine for restoring mucosal immunity in older persons. Further, our nasal pFL adjuvant system with phosphorylcholine-keyhole limpet hemocyanin (PC-KLH) has also been used in pneumococcal vaccine development to induce complete protection from nasal carriage by Streptococcus pneumoniae. Finally, the possibility that anti-PC antibodies induced by nasal delivery of pFL plus PC-KLH may play a protective role in prevention of atherogenesis and thus block subsequent development of cardiovascular disease is discussed.
Keywords: DNA-based adjuvants; Streptococcus pneumoniae; dendritic cells; nasal vaccination.
© 2017 The Societies and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest for this article.
Figures


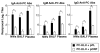
Similar articles
-
Mucosal immune features to phosphorylcholine by nasal Flt3 ligand cDNA-based vaccination.Vaccine. 2011 Aug 5;29(34):5747-57. doi: 10.1016/j.vaccine.2011.05.097. Epub 2011 Jun 15. Vaccine. 2011. PMID: 21683111
-
A combination of Flt3 ligand cDNA and CpG oligodeoxynucleotide as nasal adjuvant elicits protective secretory-IgA immunity to Streptococcus pneumoniae in aged mice.J Immunol. 2011 Feb 15;186(4):2454-61. doi: 10.4049/jimmunol.1002837. Epub 2011 Jan 17. J Immunol. 2011. PMID: 21242514
-
The nasal dendritic cell-targeting Flt3 ligand as a safe adjuvant elicits effective protection against fatal pneumococcal pneumonia.Infect Immun. 2011 Jul;79(7):2819-28. doi: 10.1128/IAI.01360-10. Epub 2011 May 2. Infect Immun. 2011. PMID: 21536790 Free PMC article.
-
The mucosal immune system of the upper respiratory tract and recent progress in mucosal vaccines.Auris Nasus Larynx. 2022 Feb;49(1):1-10. doi: 10.1016/j.anl.2021.07.003. Epub 2021 Jul 23. Auris Nasus Larynx. 2022. PMID: 34304944 Review.
-
The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae.Vaccine. 2000 Dec 8;19 Suppl 1:S87-95. doi: 10.1016/s0264-410x(00)00285-1. Vaccine. 2000. PMID: 11163470 Review.
Cited by
-
B Cell Immunosenescence.Annu Rev Cell Dev Biol. 2020 Oct 6;36:551-574. doi: 10.1146/annurev-cellbio-011620-034148. Annu Rev Cell Dev Biol. 2020. PMID: 33021823 Free PMC article. Review.
-
Synthetic CpG-ODN rapidly enriches immune compartments in neonatal chicks to induce protective immunity against bacterial infections.Sci Rep. 2019 Jan 23;9(1):341. doi: 10.1038/s41598-018-36588-6. Sci Rep. 2019. PMID: 30674918 Free PMC article.
-
Human salivary protein-derived peptides specific-salivary SIgA antibodies enhanced by nasal double DNA adjuvant in mice play an essential role in preventing Porphyromonas gingivalis colonization: an in-vitro study.BMC Oral Health. 2023 Feb 24;23(1):123. doi: 10.1186/s12903-023-02821-6. BMC Oral Health. 2023. PMID: 36829152 Free PMC article.
-
Nasal double DNA adjuvant induces salivary FimA-specific secretory IgA antibodies in young and aging mice and blocks Porphyromonas gingivalis binding to a salivary protein.BMC Oral Health. 2019 Aug 19;19(1):188. doi: 10.1186/s12903-019-0886-2. BMC Oral Health. 2019. PMID: 31426773 Free PMC article.
-
Construction of a T7 phage display nanobody library for bio-panning and identification of chicken dendritic cell-specific binding nanobodies.Sci Rep. 2022 Jul 15;12(1):12122. doi: 10.1038/s41598-022-16378-x. Sci Rep. 2022. PMID: 35840654 Free PMC article.
References
-
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, Cherian T. Hib and pneumococcal global burden of disease study team. Burden of diseases caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. - PubMed
-
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CG, Moore MR. Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. - PubMed
-
- Fukuyama Y, King JD, Kataoka K, Kobayashi R, Gilbert RS, Oishi K, Hollingshead SK, Briles DE, Fujihashi K. Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. J Immunol. 2010;185:1755–1762. - PubMed
-
- Fujihashi K, Boyaka PN, McGhee JR. Host defenses at mucosal surfaces. In: Rich RT, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM, editors. Clinical Immunology. Philadelphia, PA: Elsevier; 2013. pp. 239–251.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

