RIPK3-driven cell death during virus infections
- PMID: 28462524
- PMCID: PMC7537726
- DOI: 10.1111/imr.12539
RIPK3-driven cell death during virus infections
Abstract
The programmed self-destruction of infected cells is a powerful antimicrobial strategy in metazoans. For decades, apoptosis represented the dominant mechanism by which the virus-infected cell was thought to undergo programmed cell death. More recently, however, new mechanisms of cell death have been described that are also key to host defense. One such mechanism in vertebrates is programmed necrosis, or "necroptosis", driven by receptor-interacting protein kinase 3 (RIPK3). Once activated by innate immune stimuli, including virus infections, RIPK3 phosphorylates the mixed lineage kinase domain-like protein (MLKL), which then disrupts cellular membranes to effect necroptosis. Emerging evidence demonstrates that RIPK3 can also mediate apoptosis and regulate inflammasomes. Here, we review studies on the mechanisms by which viruses activate RIPK3 and the pathways engaged by RIPK3 that drive cell death.
Keywords: RIPK1; RIPK3; apoptosis; necroptosis; necrosis; viruses.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

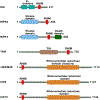
Similar articles
-
Opposite Effects of Apoptotic and Necroptotic Cellular Pathways on Rotavirus Replication.J Virol. 2022 Jan 12;96(1):e0122221. doi: 10.1128/JVI.01222-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668777 Free PMC article.
-
The neurotoxicant PCB-95 by increasing the neuronal transcriptional repressor REST down-regulates caspase-8 and increases Ripk1, Ripk3 and MLKL expression determining necroptotic neuronal death.Biochem Pharmacol. 2017 Oct 15;142:229-241. doi: 10.1016/j.bcp.2017.06.135. Epub 2017 Jul 1. Biochem Pharmacol. 2017. PMID: 28676433
-
Viral MLKL Homologs Subvert Necroptotic Cell Death by Sequestering Cellular RIPK3.Cell Rep. 2019 Sep 24;28(13):3309-3319.e5. doi: 10.1016/j.celrep.2019.08.055. Cell Rep. 2019. PMID: 31553902
-
Programmed necrosis in the cross talk of cell death and inflammation.Annu Rev Immunol. 2015;33:79-106. doi: 10.1146/annurev-immunol-032414-112248. Epub 2014 Dec 10. Annu Rev Immunol. 2015. PMID: 25493335 Free PMC article. Review.
-
Necroptosis-independent signaling by the RIP kinases in inflammation.Cell Mol Life Sci. 2016 Jun;73(11-12):2325-34. doi: 10.1007/s00018-016-2203-4. Epub 2016 Apr 5. Cell Mol Life Sci. 2016. PMID: 27048814 Free PMC article. Review.
Cited by
-
Influenza-Induced Oxidative Stress Sensitizes Lung Cells to Bacterial-Toxin-Mediated Necroptosis.Cell Rep. 2020 Aug 25;32(8):108062. doi: 10.1016/j.celrep.2020.108062. Cell Rep. 2020. PMID: 32846120 Free PMC article.
-
RNA viruses promote activation of the NLRP3 inflammasome through cytopathogenic effect-induced potassium efflux.Cell Death Dis. 2019 Apr 25;10(5):346. doi: 10.1038/s41419-019-1579-0. Cell Death Dis. 2019. PMID: 31024004 Free PMC article.
-
RIP3 Associates with RIP1, TRIF, MAVS, and Also IRF3/7 in Host Innate Immune Signaling in Large Yellow Croaker Larimichthys crocea.Antibiotics (Basel). 2021 Oct 1;10(10):1199. doi: 10.3390/antibiotics10101199. Antibiotics (Basel). 2021. PMID: 34680780 Free PMC article.
-
ZBP1/DAI Drives RIPK3-Mediated Cell Death Induced by IFNs in the Absence of RIPK1.J Immunol. 2019 Sep 1;203(5):1348-1355. doi: 10.4049/jimmunol.1900216. Epub 2019 Jul 29. J Immunol. 2019. PMID: 31358656 Free PMC article.
-
CSFV restricts necroptosis to sustain infection by inducing autophagy/mitophagy-targeted degradation of RIPK3.Microbiol Spectr. 2024 Jan 11;12(1):e0275823. doi: 10.1128/spectrum.02758-23. Epub 2023 Dec 15. Microbiol Spectr. 2024. PMID: 38100396 Free PMC article.
References
-
- Yatim N, Albert ML. Dying to replicate: the orchestration of the viral life cycle, cell death pathways, and immunity. Immunity.2011;35:478–490. - PubMed
-
- Green DR. Apoptotic pathways: the roads to ruin. Cell.1998;94:695–698. - PubMed
-
- Hardwick JM. Viral interference with apoptosis. Seminars in cell & developmental biology.1998;9:339–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

