Membrane and luminal proteins reach the apicoplast by different trafficking pathways in the malaria parasite Plasmodium falciparum
- PMID: 28462015
- PMCID: PMC5410153
- DOI: 10.7717/peerj.3128
Membrane and luminal proteins reach the apicoplast by different trafficking pathways in the malaria parasite Plasmodium falciparum
Abstract
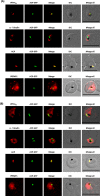
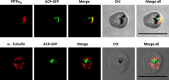
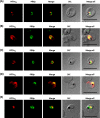
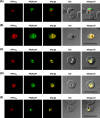
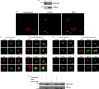
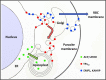
The secretory pathway in Plasmodium falciparum has evolved to transport proteins to the host cell membrane and to an endosymbiotic organelle, the apicoplast. The latter can occur via the ER or the ER-Golgi route. Here, we study these three routes using proteins Erythrocyte Membrane Protein-1 (PfEMP1), Acyl Carrier Protein (ACP) and glutathione peroxidase-like thioredoxin peroxidase (PfTPxGl) and inhibitors of vesicular transport. As expected, the G protein-dependent vesicular fusion inhibitor AlF4- and microtubule destabilizing drug vinblastine block the trafficking of PfEMP-1, a protein secreted to the host cell membrane. However, while both PfTPxGl and ACP are targeted to the apicoplast, only ACP trafficking remains unaffected by these treatments. This implies that G protein-dependent vesicles do not play a role in classical apicoplast protein targeting. Unlike the soluble protein ACP, we show that PfTPxGl is localized to the outermost membrane of the apicoplast. Thus, the parasite apicoplast acquires proteins via two different pathways: first, the vesicular trafficking pathway appears to handle not only secretory proteins, but an apicoplast membrane protein, PfTPxGl; second, trafficking of apicoplast luminal proteins appear to be independent of G protein-coupled vesicles.
Keywords: Acyl-carrier protein (ACP-GFP); Apicoplast; Glutathione peroxidase like thioredoxin peroxidase (PfTPxGl); P. falciparum; Protein trafficking.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

Similar articles
-
Heterologous expression in Toxoplasma gondii reveals a topogenic signal anchor in a Plasmodium apicoplast protein.FEBS Open Bio. 2018 Oct 22;8(11):1746-1762. doi: 10.1002/2211-5463.12527. eCollection 2018 Nov. FEBS Open Bio. 2018. PMID: 30410855 Free PMC article.
-
Protein Sorting in Plasmodium Falciparum.Life (Basel). 2021 Sep 9;11(9):937. doi: 10.3390/life11090937. Life (Basel). 2021. PMID: 34575086 Free PMC article. Review.
-
Evidence for Golgi-independent transport from the early secretory pathway to the plastid in malaria parasites.Mol Microbiol. 2006 Aug;61(3):614-30. doi: 10.1111/j.1365-2958.2006.05244.x. Epub 2006 Jun 20. Mol Microbiol. 2006. PMID: 16787449
-
Protein Traffic to the Plasmodium falciparum apicoplast: evidence for a sorting branch point at the Golgi.Traffic. 2014 Dec;15(12):1290-304. doi: 10.1111/tra.12226. Epub 2014 Oct 15. Traffic. 2014. PMID: 25264207
-
Protein targeting to the malaria parasite plastid.Traffic. 2008 Feb;9(2):166-75. doi: 10.1111/j.1600-0854.2007.00660.x. Epub 2007 Nov 13. Traffic. 2008. PMID: 17900270 Review.
Cited by
-
Heterologous expression in Toxoplasma gondii reveals a topogenic signal anchor in a Plasmodium apicoplast protein.FEBS Open Bio. 2018 Oct 22;8(11):1746-1762. doi: 10.1002/2211-5463.12527. eCollection 2018 Nov. FEBS Open Bio. 2018. PMID: 30410855 Free PMC article.
-
The Dissection of SNAREs Reveals Key Factors for Vesicular Trafficking to the Endosome-like Compartment and Apicoplast via the Secretory System in Toxoplasma gondii.mBio. 2021 Aug 31;12(4):e0138021. doi: 10.1128/mBio.01380-21. Epub 2021 Aug 3. mBio. 2021. PMID: 34340555 Free PMC article.
-
The Potential use of a Curcumin-Piperine Combination as an Antimalarial Agent: A Systematic Review.J Trop Med. 2021 Oct 11;2021:9135617. doi: 10.1155/2021/9135617. eCollection 2021. J Trop Med. 2021. PMID: 34671402 Free PMC article. Review.
-
Protein Sorting in Plasmodium Falciparum.Life (Basel). 2021 Sep 9;11(9):937. doi: 10.3390/life11090937. Life (Basel). 2021. PMID: 34575086 Free PMC article. Review.
-
The ZIP Code of Vesicle Trafficking in Apicomplexa: SEC1/Munc18 and SNARE Proteins.mBio. 2020 Oct 20;11(5):e02092-20. doi: 10.1128/mBio.02092-20. mBio. 2020. PMID: 33082261 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

