Using patient values and preferences to inform the importance of health outcomes in practice guideline development following the GRADE approach
- PMID: 28460638
- PMCID: PMC5412036
- DOI: 10.1186/s12955-017-0621-0
Using patient values and preferences to inform the importance of health outcomes in practice guideline development following the GRADE approach
Abstract
Background: There are diverse opinions and confusion about defining and including patient values and preferences (i.e. the importance people place on the health outcomes) in the guideline development processes. This article aims to provide an overview of a process for systematically incorporating values and preferences in guideline development.
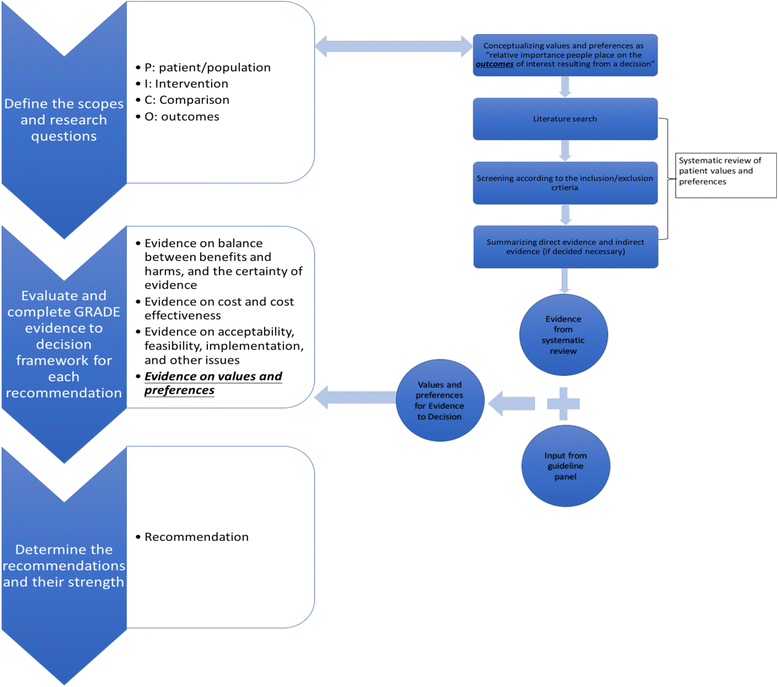
Methods: In 2013 and 2014, we followed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to adopt, adapt and develop 226 recommendations in 22 guidelines for the Ministry of Health of the Kingdom of Saudi Arabia. To collect context-specific values and preferences for each recommendation, we performed systematic reviews, asked clinical experts to provide feedback according to their clinical experience, and consulted patient representatives.
Results: We found several types of studies addressing the importance of outcomes, including those reporting utilities, non-utility measures of health states based on structured questionnaires or scales, and qualitative studies. Guideline panels used the relative importance of outcomes based on values and preferences to weigh the balance of desirable and undesirable consequences of alternative intervention options. However, we found few studies addressing local values and preferences.
Conclusions: Currently there are different but no firmly established processes for integrating patient values and preferences in healthcare decision-making of practice guideline development. With GRADE Evidence-to-Decision (EtD) frameworks, we provide an empirical strategy to find and incorporate values and preferences in guidelines by performing systematic reviews and eliciting information from guideline panel members and patient representatives. However, more research and practical guidance are needed on how to search for relevant studies and grey literature, assess the certainty of this evidence, and best summarize and present the findings.
Keywords: Evidence to decision; Guideline development; Outcome importance; Patient preferences; Patient values; Systematic review.
Figures
Similar articles
-
Synthesis, grading, and presentation of evidence in guidelines: article 7 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report.Proc Am Thorac Soc. 2012 Dec;9(5):256-61. doi: 10.1513/pats.201208-060ST. Proc Am Thorac Soc. 2012. PMID: 23256168 Review.
-
Deciding what type of evidence and outcomes to include in guidelines: article 5 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report.Proc Am Thorac Soc. 2012 Dec;9(5):243-50. doi: 10.1513/pats.201208-058ST. Proc Am Thorac Soc. 2012. PMID: 23256166 Review.
-
Moving from evidence to developing recommendations in guidelines: article 11 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report.Proc Am Thorac Soc. 2012 Dec;9(5):282-92. doi: 10.1513/pats.201208-064ST. Proc Am Thorac Soc. 2012. PMID: 23256172 Review.
-
GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT.J Clin Epidemiol. 2017 Jan;81:101-110. doi: 10.1016/j.jclinepi.2016.09.009. Epub 2016 Oct 3. J Clin Epidemiol. 2017. PMID: 27713072
-
[GRADE: Using evidence to decision frameworks for adoption, adaptation and de novo generation of trustworthy guideline recommendations - GRADE-ADOLOPMENT].Z Evid Fortbild Qual Gesundhwes. 2019 Aug;144-145:90-99. doi: 10.1016/j.zefq.2019.06.001. Epub 2019 Aug 6. Z Evid Fortbild Qual Gesundhwes. 2019. PMID: 31399391 German.
Cited by
-
Composite to Clarity: Shifting From Combined to Individual Endpoints in Meta-Analyses of Cardiovascular Outcome Trials.JACC Adv. 2023 Aug 6;2(7):100548. doi: 10.1016/j.jacadv.2023.100548. eCollection 2023 Sep. JACC Adv. 2023. PMID: 38939474 Free PMC article. No abstract available.
-
Diabetes patient preferences for glucose-monitoring technologies: results from a discrete choice experiment in Poland and the Netherlands.BMJ Open Diabetes Res Care. 2023 Jan;11(1):e003025. doi: 10.1136/bmjdrc-2022-003025. BMJ Open Diabetes Res Care. 2023. PMID: 36649973 Free PMC article.
-
α1-Acid Glycoprotein and Dietary Intake in End-Stage Renal Disease Patients.Nutrients. 2021 Oct 20;13(11):3671. doi: 10.3390/nu13113671. Nutrients. 2021. PMID: 34835927 Free PMC article.
-
Use of the ADAPTE method to develop a clinical guideline for the improvement of psychoses and schizophrenia care: Example of involvement and participation of patients and family caregivers.Health Expect. 2021 Apr;24(2):516-524. doi: 10.1111/hex.13193. Epub 2021 Feb 23. Health Expect. 2021. PMID: 33621426 Free PMC article.
-
Patient preferences for breast cancer screening: a systematic review update to inform recommendations by the Canadian Task Force on Preventive Health Care.Syst Rev. 2024 May 28;13(1):140. doi: 10.1186/s13643-024-02539-8. Syst Rev. 2024. PMID: 38807191 Free PMC article.
References
-
- World Health Organization . WHO Handbook for Guideline Development. 2. Switzerland: World Health Organization; 2014.
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. - DOI - PMC - PubMed
-
- Schunemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, Ventresca M, Brignardello-Petersen R, Laisaar KT, Kowalski S, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014;186:E123–142. doi: 10.1503/cmaj.131237. - DOI - PMC - PubMed
-
- Schunemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, Scholten R, Langendam M, Leeflang MM, Akl EA, et al. Development of the GRADE Evidence to Decision (EtD) frameworks for tests in clinical practice and public health. J Clin Epidemiol. 2016;76:89-98. doi: 10.1016/j.jclinepi.2016.01.032. - PubMed
-
- Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

