KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma
- PMID: 28451792
- PMCID: PMC5579171
- DOI: 10.1007/s00262-017-2005-z
KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma
Abstract
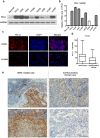
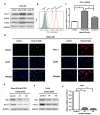
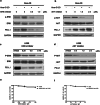
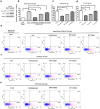
It was reported that PD-L1 expression was correlated with genetic alterations. Whether PD-L1 was regulated by mutant Kirsten rat sarcoma viral oncogene homolog (KRAS) in non-small-cell lung cancer (NSCLC) and the underlying molecular mechanism were largely unknown. In this study, we investigated the correlation between PD-L1 expression and KRAS mutation and the functional significance of PD-1/PD-L1 blockade in KRAS-mutant lung adenocarcinoma. We found that PD-L1 expression was associated with KRAS mutation both in the human lung adenocarcinoma cell lines and tissues. PD-L1 was up-regulated by KRAS mutation through p-ERK but not p-AKT signaling. We also found that KRAS-mediated up-regulation of PD-L1 induced the apoptosis of CD3-positive T cells which was reversed by anti-PD-1 antibody (Pembrolizumab) or ERK inhibitor. PD-1 blocker or ERK inhibitor could recover the anti-tumor immunity of T cells and decrease the survival rates of KRAS-mutant NSCLC cells in co-culture system in vitro. However, Pembrolizumab combined with ERK inhibitor did not show synergistic effect on killing tumor cells in co-culture system. Our study demonstrated that KRAS mutation could induce PD-L1 expression through p-ERK signaling in lung adenocarcinoma. Blockade of PD-1/PD-L1 pathway may be a promising therapeutic strategy for human KRAS-mutant lung adenocarcinoma.
Keywords: KRAS; Lung adenocarcinoma; PD-1; PD-L1.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer.Cancer Commun (Lond). 2022 Sep;42(9):828-847. doi: 10.1002/cac2.12327. Epub 2022 Jul 11. Cancer Commun (Lond). 2022. PMID: 35811500 Free PMC article.
-
The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity.Cancer Lett. 2020 Feb 1;470:95-105. doi: 10.1016/j.canlet.2019.10.027. Epub 2019 Oct 20. Cancer Lett. 2020. PMID: 31644929
-
Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma.Clin Cancer Res. 2017 Jun 15;23(12):3012-3024. doi: 10.1158/1078-0432.CCR-16-2554. Epub 2016 Dec 30. Clin Cancer Res. 2017. PMID: 28039262
-
PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review.Lung Cancer. 2017 Oct;112:200-215. doi: 10.1016/j.lungcan.2017.08.005. Epub 2017 Aug 10. Lung Cancer. 2017. PMID: 29191596 Review.
-
Targeting the KRAS variant for treatment of non-small cell lung cancer: potential therapeutic applications.Expert Rev Respir Med. 2016;10(1):53-68. doi: 10.1586/17476348.2016.1115349. Epub 2015 Nov 17. Expert Rev Respir Med. 2016. PMID: 26714748 Review.
Cited by
-
Programmed Death-Ligand 1 Expression in Lung Cancer and Paired Brain Metastases-a Single-Center Study in 190 Patients.JTO Clin Res Rep. 2022 Sep 20;3(11):100413. doi: 10.1016/j.jtocrr.2022.100413. eCollection 2022 Nov. JTO Clin Res Rep. 2022. PMID: 36275910 Free PMC article.
-
Complete response to nivolumab in Kirsten rat sarcoma virus oncogene KRAS-G12C mutant metastatic lung adenocarcinoma: a case report.J Med Case Rep. 2022 Nov 3;16(1):420. doi: 10.1186/s13256-022-03593-3. J Med Case Rep. 2022. PMID: 36329437 Free PMC article.
-
Tumor-intrinsic signaling pathways: key roles in the regulation of the immunosuppressive tumor microenvironment.J Hematol Oncol. 2019 Nov 27;12(1):125. doi: 10.1186/s13045-019-0804-8. J Hematol Oncol. 2019. PMID: 31775797 Free PMC article. Review.
-
Onco-immunomodulatory properties of pharmacological interference with RAS-RAF-MEK-ERK pathway hyperactivation.Front Oncol. 2022 Jul 27;12:931774. doi: 10.3389/fonc.2022.931774. eCollection 2022. Front Oncol. 2022. PMID: 35965494 Free PMC article. Review.
-
The Mechanisms of PD-L1 Regulation in Non-Small-Cell Lung Cancer (NSCLC): Which Are the Involved Players?Cancers (Basel). 2020 Oct 26;12(11):3129. doi: 10.3390/cancers12113129. Cancers (Basel). 2020. PMID: 33114576 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 81372502/Chinese National Natural Science Foundation
- 81601991/Chinese National Natural Science Foundation
- HN2014-05/Open project of State Key laboratory of Oncology in South China
- A2016203/Medical Scientific Research Fund of Guangdong Province
- 14ykpy38/Young Teacher Training Program of Sun Yat-Sen University
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

