Galectin-3 Is a Target for Proteases Involved in the Virulence of Staphylococcus aureus
- PMID: 28438975
- PMCID: PMC5478954
- DOI: 10.1128/IAI.00177-17
Galectin-3 Is a Target for Proteases Involved in the Virulence of Staphylococcus aureus
Abstract
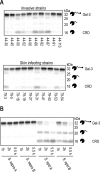
Staphylococcus aureus is a major cause of skin and soft tissue infection. The bacterium expresses four major proteases that are emerging as virulence factors: aureolysin (Aur), V8 protease (SspA), staphopain A (ScpA), and staphopain B (SspB). We hypothesized that human galectin-3, a β-galactoside-binding lectin involved in immune regulation and antimicrobial defense, is a target for these proteases and that proteolysis of galectin-3 is a novel immune evasion mechanism. Indeed, supernatants from laboratory strains and clinical isolates of S. aureus caused galectin-3 degradation. Similar proteolytic capacities were found in Staphylococcus epidermidis isolates but not in Staphylococcus saprophyticus Galectin-3-induced activation of the neutrophil NADPH oxidase was abrogated by bacterium-derived proteolysis of galectin-3, and SspB was identified as the major protease responsible. The impact of galectin-3 and protease expression on S. aureus virulence was studied in a murine skin infection model. In galectin-3+/+ mice, SspB-expressing S. aureus caused larger lesions and resulted in higher bacterial loads than protease-lacking bacteria. No such difference in bacterial load or lesion size was detected in galectin-3-/- mice, which overall showed smaller lesion sizes than the galectin-3+/+ animals. In conclusion, the staphylococcal protease SspB inactivates galectin-3, abrogating its stimulation of oxygen radical production in human neutrophils and increasing tissue damage during skin infection.
Keywords: Staphylococcus aureus; galectin-3; neutrophils; protease; skin infection; staphopain; virulence; virulence factors; virulence regulation.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Increased production of aureolysin and staphopain A is a primary determinant of the reduced virulence of Staphylococcus aureus sarA mutants in osteomyelitis.mBio. 2024 Apr 10;15(4):e0338323. doi: 10.1128/mbio.03383-23. Epub 2024 Feb 28. mBio. 2024. PMID: 38415646 Free PMC article.
-
The role and regulation of the extracellular proteases of Staphylococcus aureus.Microbiology (Reading). 2004 Jan;150(Pt 1):217-228. doi: 10.1099/mic.0.26634-0. Microbiology (Reading). 2004. PMID: 14702415
-
Impact of individual extracellular proteases on Staphylococcus aureus biofilm formation in diverse clinical isolates and their isogenic sarA mutants.Microbiologyopen. 2014 Dec;3(6):897-909. doi: 10.1002/mbo3.214. Epub 2014 Sep 25. Microbiologyopen. 2014. PMID: 25257373 Free PMC article.
-
Staphylococcus aureus Manipulates Innate Immunity through Own and Host-Expressed Proteases.Front Cell Infect Microbiol. 2017 May 5;7:166. doi: 10.3389/fcimb.2017.00166. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28529927 Free PMC article. Review.
-
Papain-like proteases of Staphylococcus aureus.Adv Exp Med Biol. 2011;712:1-14. doi: 10.1007/978-1-4419-8414-2_1. Adv Exp Med Biol. 2011. PMID: 21660655 Review.
Cited by
-
Staphylococcus aureus Proteases: Orchestrators of Skin Inflammation.DNA Cell Biol. 2024 Oct;43(10):483-491. doi: 10.1089/dna.2024.0134. Epub 2024 Jul 3. DNA Cell Biol. 2024. PMID: 38957987 Review.
-
The Sweet-Side of Leukocytes: Galectins as Master Regulators of Neutrophil Function.Front Immunol. 2019 Aug 7;10:1762. doi: 10.3389/fimmu.2019.01762. eCollection 2019. Front Immunol. 2019. PMID: 31440233 Free PMC article. Review.
-
The Role of Collectins and Galectins in Lung Innate Immune Defense.Front Immunol. 2018 Sep 4;9:1998. doi: 10.3389/fimmu.2018.01998. eCollection 2018. Front Immunol. 2018. PMID: 30233589 Free PMC article. Review.
-
Comparative secretome analysis of Staphylococcus aureus strains with different within-herd intramammary infection prevalence.Virulence. 2022 Dec;13(1):174-190. doi: 10.1080/21505594.2021.2024014. Virulence. 2022. PMID: 35030987 Free PMC article.
-
Novel inhibitory effect of galectin-3 on the respiratory burst induced by Staphylococcus aureus in human neutrophils.Glycobiology. 2023 Jun 21;33(6):503-511. doi: 10.1093/glycob/cwad032. Glycobiology. 2023. PMID: 37073717 Free PMC article.
References
-
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M, SENTRY Participants Group. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis 32(Suppl 2):S114–S132. doi:10.1086/320184. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

