RANKL cytokine enhances TNF-induced osteoclastogenesis independently of TNF receptor associated factor (TRAF) 6 by degrading TRAF3 in osteoclast precursors
- PMID: 28438834
- PMCID: PMC5473222
- DOI: 10.1074/jbc.M116.771816
RANKL cytokine enhances TNF-induced osteoclastogenesis independently of TNF receptor associated factor (TRAF) 6 by degrading TRAF3 in osteoclast precursors
Abstract
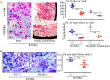
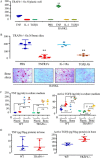
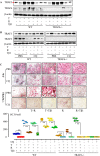
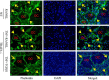
Cytokines, including receptor activator of nuclear factor κB ligand (RANKL) and TNF, induce increased osteoclast (OC) formation and bone loss in postmenopausal osteoporosis and inflammatory arthritides. RANKL and TNF can independently induce OC formation in vitro from WT OC precursors via TNF receptor-associated factor (TRAF) adaptor proteins, which bind to their receptors. Of these, only TRAF6 is required for RANKL-induced osteoclastogenesis in vitro However, the molecular mechanisms involved remain incompletely understood. Here we report that RANKL induced the formation of bone-resorbing OCs from TRAF6-/- OC precursors when cultured on bone slices but not on plastic. The mechanisms involved increased TNF production by TRAF6-/- OC precursors resulting from their interaction with bone matrix and release of active TGFβ from the resorbed bone, coupled with RANKL-induced autophagolysosomal degradation of TRAF3, a known inhibitor of OC formation. Consistent with these findings, RANKL enhanced TNF-induced OC formation from TRAF6-/- OC precursors. Moreover, TNF induced significantly more OCs from mice with TRAF3 conditionally deleted in myeloid lineage cells, and it did not inhibit RANKL-induced OC formation from these cells. TRAF6-/- OC precursors that overexpressed TRAF3 or were treated with the autophagolysosome inhibitor chloroquine formed significantly fewer OCs in response to TNF alone or in combination with RANKL. We conclude that RANKL can enhance TNF-induced OC formation independently of TRAF6 by degrading TRAF3. These findings suggest that preventing TRAF3 degradation with drugs like chloroquine could reduce excessive OC formation in diseases in which bone resorption is increased in response to elevated production of these cytokines.
Keywords: RANKL; TGF-β; TNF; TNF receptor-associated factor (TRAF); bone; osteoclast.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation.J Clin Invest. 2014 Jan;124(1):297-310. doi: 10.1172/JCI66947. Epub 2013 Dec 9. J Clin Invest. 2014. PMID: 24316970 Free PMC article.
-
Bone Remodeling and the Role of TRAF3 in Osteoclastic Bone Resorption.Front Immunol. 2018 Sep 28;9:2263. doi: 10.3389/fimmu.2018.02263. eCollection 2018. Front Immunol. 2018. PMID: 30323820 Free PMC article. Review.
-
Nuclear Factor-Kappa B Regulation of Osteoclastogenesis and Osteoblastogenesis.Endocrinol Metab (Seoul). 2023 Oct;38(5):504-521. doi: 10.3803/EnM.2023.501. Epub 2023 Sep 26. Endocrinol Metab (Seoul). 2023. PMID: 37749800 Free PMC article.
-
Rictor Is a Novel Regulator of TRAF6/TRAF3 in Osteoclasts.J Bone Miner Res. 2021 Oct;36(10):2053-2064. doi: 10.1002/jbmr.4398. Epub 2021 Jul 1. J Bone Miner Res. 2021. PMID: 34155681
-
Regulation of TNF-Induced Osteoclast Differentiation.Cells. 2021 Dec 31;11(1):132. doi: 10.3390/cells11010132. Cells. 2021. PMID: 35011694 Free PMC article. Review.
Cited by
-
Roles of the RANKL-RANK Axis in Immunity-Implications for Pathogenesis and Treatment of Bone Metastasis.Front Immunol. 2022 Mar 21;13:824117. doi: 10.3389/fimmu.2022.824117. eCollection 2022. Front Immunol. 2022. PMID: 35386705 Free PMC article. Review.
-
Anti-Inflammatory Effects of Adenine Enhance Osteogenesis in the Osteoblast-Like MG-63 Cells.Life (Basel). 2020 Jul 19;10(7):116. doi: 10.3390/life10070116. Life (Basel). 2020. PMID: 32707735 Free PMC article.
-
Lipopolysaccharide sensitizes the therapeutic response of breast cancer to IAP antagonist.Front Immunol. 2022 Aug 31;13:906357. doi: 10.3389/fimmu.2022.906357. eCollection 2022. Front Immunol. 2022. PMID: 36119107 Free PMC article.
-
Osteoclastogenesis in periodontal diseases: Possible mediators and mechanisms.J Oral Biosci. 2020 Jun;62(2):123-130. doi: 10.1016/j.job.2020.02.002. Epub 2020 Feb 17. J Oral Biosci. 2020. PMID: 32081710 Free PMC article. Review.
-
The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: a review.Front Immunol. 2023 Jul 5;14:1222129. doi: 10.3389/fimmu.2023.1222129. eCollection 2023. Front Immunol. 2023. PMID: 37475866 Free PMC article. Review.
References
-
- Kim H. H., Lee D. E., Shin J. N., Lee Y. S., Jeon Y. M., Chung C. H., Ni J., Kwon B. S., and Lee Z. H. (1999) Receptor activator of NF-κB recruits multiple TRAF family adaptors and activates c-Jun N-terminal kinase. FEBS Lett. 443, 297–302 - PubMed
-
- Wong B. R., Josien R., Lee S. Y., Vologodskaia M., Steinman R. M., and Choi Y. (1998) The TRAF family of signal transducers mediates NF-κB activation by the TRANCE receptor. J. Biol. Chem. 273, 28355–28359 - PubMed
-
- Galibert L., Tometsko M. E., Anderson D. M., Cosman D., and Dougall W. C. (1998) The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-κB, a member of the TNFR superfamily. J. Biol. Chem. 273, 34120–34127 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

