Conformational dynamics of bacterial trigger factor in apo and ribosome-bound states
- PMID: 28437479
- PMCID: PMC5402958
- DOI: 10.1371/journal.pone.0176262
Conformational dynamics of bacterial trigger factor in apo and ribosome-bound states
Abstract
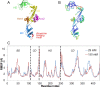
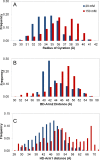
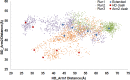
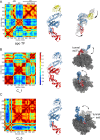
The chaperone trigger factor (TF) binds to the ribosome exit tunnel and helps cotranslational folding of nascent chains (NC) in bacterial cells and chloroplasts. In this study, we aim to investigate the functional dynamics of fully-atomistic apo TF and its complex with 50S. As TF accomodates a high percentage of charged residues on its surface, the effect of ionic strength on TF dynamics is assessed here by performing five independent molecular dynamics (MD) simulations (total of 1.3 micro-second duration) at 29 mM and 150 mM ionic strengths. At both concentrations, TF exhibits high inter- and intra-domain flexibility related to its binding (BD), core (CD), and head (HD) domains. Even though large oscillations in gyration radius exist during each run, we do not detect the so-called 'fully collapsed' state with both HD and BD collapsed upon the core. In fact, the extended conformers with relatively open HD and BD are highly populated at the physiological concentration of 150 mM. More importantly, extended TF snapshots stand out in terms of favorable docking onto the 50S subunit. Elastic network modeling (ENM) indicates significant changes in TF's functional dynamics and domain decomposition depending on its conformation and positioning on the 50S. The most dominant slow motions are the lateral sweeping and vertical opening/closing of HD relative to 50S. Finally, our ENM-based efficient technique -ClustENM- is used to sample atomistic conformers starting with an extended TF-50S complex. Specifically, BD and CD motions are restricted near the tunnel exit, while HD is highly mobile. The atomistic conformers generated without an NC are in agreement with the cryo-EM maps available for TF-ribosome-NC complex.
Conflict of interest statement
Figures
Similar articles
-
Dynamic Behavior of Trigger Factor on the Ribosome.J Mol Biol. 2016 Sep 11;428(18):3588-602. doi: 10.1016/j.jmb.2016.06.007. Epub 2016 Jun 16. J Mol Biol. 2016. PMID: 27320387
-
Localization of the trigger factor binding site on the ribosomal 50S subunit.J Mol Biol. 2003 Feb 21;326(3):887-97. doi: 10.1016/s0022-2836(02)01436-5. J Mol Biol. 2003. PMID: 12581648
-
Structure of trigger factor binding domain in biologically homologous complex with eubacterial ribosome reveals its chaperone action.Proc Natl Acad Sci U S A. 2005 Aug 23;102(34):12017-22. doi: 10.1073/pnas.0505581102. Epub 2005 Aug 9. Proc Natl Acad Sci U S A. 2005. PMID: 16091460 Free PMC article.
-
A cradle for new proteins: trigger factor at the ribosome.Curr Opin Struct Biol. 2005 Apr;15(2):204-12. doi: 10.1016/j.sbi.2005.03.005. Curr Opin Struct Biol. 2005. PMID: 15837180 Review.
-
Cotranslational processing mechanisms: towards a dynamic 3D model.Trends Biochem Sci. 2009 Aug;34(8):417-26. doi: 10.1016/j.tibs.2009.04.003. Epub 2009 Jul 31. Trends Biochem Sci. 2009. PMID: 19647435 Review.
Cited by
-
Towards gaining sight of multiscale events: utilizing network models and normal modes in hybrid methods.Curr Opin Struct Biol. 2020 Oct;64:34-41. doi: 10.1016/j.sbi.2020.05.013. Epub 2020 Jul 1. Curr Opin Struct Biol. 2020. PMID: 32622329 Free PMC article. Review.
-
Pre- and post-docking sampling of conformational changes using ClustENM and HADDOCK for protein-protein and protein-DNA systems.Proteins. 2020 Feb;88(2):292-306. doi: 10.1002/prot.25802. Epub 2019 Sep 3. Proteins. 2020. PMID: 31441121 Free PMC article.
-
Intrinsic dynamics is evolutionarily optimized to enable allosteric behavior.Curr Opin Struct Biol. 2020 Jun;62:14-21. doi: 10.1016/j.sbi.2019.11.002. Epub 2019 Nov 27. Curr Opin Struct Biol. 2020. PMID: 31785465 Free PMC article. Review.
-
Sampling of Protein Conformational Space Using Hybrid Simulations: A Critical Assessment of Recent Methods.Front Mol Biosci. 2022 Feb 4;9:832847. doi: 10.3389/fmolb.2022.832847. eCollection 2022. Front Mol Biosci. 2022. PMID: 35187088 Free PMC article.
-
The Dynamic SecYEG Translocon.Front Mol Biosci. 2021 Apr 15;8:664241. doi: 10.3389/fmolb.2021.664241. eCollection 2021. Front Mol Biosci. 2021. PMID: 33937339 Free PMC article. Review.
References
-
- Kramer G, Rauch T, Rist W, Vorderwülbecke S, Patzelt H, Schulze-Specking A, et al. L23 protein functions as a chaperone docking site on the ribosome. Nature. 2002;419: 171–174. doi: 10.1038/nature01047 - DOI - PubMed
-
- Ferbitz L, Maier T, Patzelt H, Bukau B, Deuerling E, Ban N. Trigger factor in complex with the ribosome forms a molecular cradle for nascent proteins. Nature. 2004;431: 590–596. doi: 10.1038/nature02899 - DOI - PubMed
-
- Baram D, Pyetan E, Sittner A, Auerbach-Nevo T, Bashan A, Yonath A. Structure of trigger factor binding domain in biologically homologous complex with eubacterial ribosome reveals its chaperone action. Proc Natl Acad Sci U S A. 2005;102: 12017–12022. doi: 10.1073/pnas.0505581102 - DOI - PMC - PubMed
-
- Schlünzen F, Wilson DN, Tian P, Harms JM, McInnes SJ, Hansen HAS, et al. The binding mode of the trigger factor on the ribosome: Implications for protein folding and SRP interaction. Structure. 2005;13: 1685–1694. doi: 10.1016/j.str.2005.08.007 - DOI - PubMed
-
- Merz F, Boehringer D, Schaffitzel C, Preissler S, Hoffmann A, Maier T, et al. Molecular mechanism and structure of Trigger Factor bound to the translating ribosome. EMBO J. 2008;27: 1622–1632. doi: 10.1038/emboj.2008.89 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

