Hypermethylation of Interferon Regulatory Factor 8 (IRF8) Confers Risk to Vogt-Koyanagi-Harada Disease
- PMID: 28432342
- PMCID: PMC5430771
- DOI: 10.1038/s41598-017-01249-7
Hypermethylation of Interferon Regulatory Factor 8 (IRF8) Confers Risk to Vogt-Koyanagi-Harada Disease
Abstract
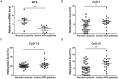
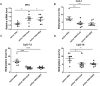
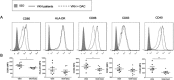
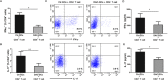
Aberrant methylation change of IRF8 confers risk to various tumors, and abnormal expression of IRF8 is involved in many autoimmune diseases, including ocular Behcet's disease. However, whether the methylation change of IRF8 is associated with Vogt-Koyanagi-Harada (VKH) disease remains unknown. In the present study, we found a decreased IRF8 mRNA expression in association with a higher methylation level in monocyte-derived dendritic cells (DCs) from active VKH patients compared with the normal and inactive subjects. DCs incubated with cyclosporin a (CsA) or dexamethasone (DEX) showed a lower methylation and higher mRNA expression of IRF8 in active VKH patients. A demethylation reagent, 5-Aza-2'-deoxycytidine (DAC) showed a notable demethylation effect as evidenced by increasing the mRNA expression and reducing the methylation level of IRF8. It also suppressed the Th1 and Th17 responses through down-regulating the expression of co-stimulatory molecules (CD86, CD80, CD40), and reducing the production of pro-inflammatory cytokines (IL-6, IL-1β, IL-23, IL-12) produced by DCs. These findings shows that hypermethylation of IRF8 in DCs confers risk to VKH disease. Demethylation of IRF8 may offer a novel therapeutic strategy protect against VKH disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Ocular Behcet's disease is associated with aberrant methylation of interferon regulatory factor 8 (IRF8) in monocyte-derived dendritic cells.Oncotarget. 2017 Apr 19;8(31):51277-51287. doi: 10.18632/oncotarget.17235. eCollection 2017 Aug 1. Oncotarget. 2017. PMID: 28881647 Free PMC article.
-
Decreased expression of A20 is associated with ocular Behcet's disease (BD) but not with Vogt-Koyanagi-Harada (VKH) disease.Br J Ophthalmol. 2018 Aug;102(8):1167-1172. doi: 10.1136/bjophthalmol-2017-311707. Epub 2018 Apr 26. Br J Ophthalmol. 2018. PMID: 29699987
-
Disabled-2 (DAB2) Overexpression Inhibits Monocyte-Derived Dendritic Cells' Function in Vogt-Koyanagi-Harada Disease.Invest Ophthalmol Vis Sci. 2018 Sep 4;59(11):4662-4669. doi: 10.1167/iovs.18-24630. Invest Ophthalmol Vis Sci. 2018. PMID: 30267088
-
The emerging role of epigenetics and gut microbiota in Vogt-Koyanagi-Harada syndrome.Gene. 2022 Apr 15;818:146222. doi: 10.1016/j.gene.2022.146222. Epub 2022 Jan 29. Gene. 2022. PMID: 35092860 Review.
-
Vogt-koyanagi-harada syndrome.Curr Eye Res. 2008 Jul;33(7):517-23. doi: 10.1080/02713680802233968. Curr Eye Res. 2008. PMID: 18600484 Review.
Cited by
-
Lower Interferon Regulatory Factor-8 Expression in Peripheral Myeloid Cells Tracks With Adverse Central Nervous System Outcomes in Treated HIV Infection.Front Immunol. 2019 Nov 29;10:2789. doi: 10.3389/fimmu.2019.02789. eCollection 2019. Front Immunol. 2019. PMID: 31849969 Free PMC article.
-
Dynamic DNA Methylation Changes of Tbx21 and Rorc during Experimental Autoimmune Uveitis in Mice.Mediators Inflamm. 2018 Sep 4;2018:9129163. doi: 10.1155/2018/9129163. eCollection 2018. Mediators Inflamm. 2018. PMID: 30254507 Free PMC article.
-
Ankylosing spondylitis is associated with aberrant DNA methylation of IFN regulatory factor 8 gene promoter region.Clin Rheumatol. 2019 Aug;38(8):2161-2169. doi: 10.1007/s10067-019-04505-5. Epub 2019 Mar 21. Clin Rheumatol. 2019. PMID: 30900036
-
FTO-mediated m6A modification alleviates autoimmune uveitis by regulating microglia phenotypes via the GPC4/TLR4/NF-κB signaling axis.Genes Dis. 2022 Oct 4;10(5):2179-2193. doi: 10.1016/j.gendis.2022.09.008. eCollection 2023 Sep. Genes Dis. 2022. PMID: 37492748 Free PMC article.
-
Epigenetic Modifications and Therapy in Uveitis.Front Cell Dev Biol. 2021 Nov 18;9:758240. doi: 10.3389/fcell.2021.758240. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34869347 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

