Genomic Characterization of Recrudescent Plasmodium malariae after Treatment with Artemether/Lumefantrine
- PMID: 28430103
- PMCID: PMC5547787
- DOI: 10.3201/eid2308.161582
Genomic Characterization of Recrudescent Plasmodium malariae after Treatment with Artemether/Lumefantrine
Abstract
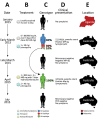
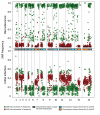
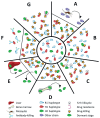
Plasmodium malariae is the only human malaria parasite species with a 72-hour intraerythrocytic cycle and the ability to persist in the host for life. We present a case of a P. malariae infection with clinical recrudescence after directly observed administration of artemether/lumefantrine. By using whole-genome sequencing, we show that the initial infection was polyclonal and the recrudescent isolate was a single clone present at low density in the initial infection. Haplotypic analysis of the clones in the initial infection revealed that they were all closely related and were presumably recombinant progeny originating from the same infective mosquito bite. We review possible explanations for the P. malariae treatment failure and conclude that a 3-day artemether/lumefantrine regimen is suboptimal for this species because of its long asexual life cycle.
Keywords: Australia; Plasmodium malariae; artemether/lumefantrine; haplotype; malaria; parasitemia; parasites; recrudescence.
Figures
Similar articles
-
Plasmodium malariae after successful treatment of P. falciparum malaria with artemether-lumefantrine.Int J Infect Dis. 2022 Jun;119:56-58. doi: 10.1016/j.ijid.2022.03.045. Epub 2022 Mar 28. Int J Infect Dis. 2022. PMID: 35358721
-
Why do Plasmodium malariae infections sometimes occur in spite of previous antimalarial medication?Parasitol Res. 2012 Aug;111(2):943-6. doi: 10.1007/s00436-012-2851-8. Epub 2012 Feb 18. Parasitol Res. 2012. PMID: 22350675
-
Evaluation of recurrent parasitemia after artemether-lumefantrine treatment for uncomplicated malaria in children in western Kenya.Am J Trop Med Hyg. 2010 Sep;83(3):458-64. doi: 10.4269/ajtmh.2010.09-0403. Am J Trop Med Hyg. 2010. PMID: 20810804 Free PMC article. Clinical Trial.
-
The use of artemether-lumefantrine for the treatment of uncomplicated Plasmodium vivax malaria.PLoS Negl Trop Dis. 2011 Dec;5(12):e1325. doi: 10.1371/journal.pntd.0001325. Epub 2011 Dec 27. PLoS Negl Trop Dis. 2011. PMID: 22216359 Free PMC article. Review.
-
The clinical efficacy of artemether/lumefantrine (Coartem).Malar J. 2009 Oct 12;8 Suppl 1(Suppl 1):S5. doi: 10.1186/1475-2875-8-S1-S5. Malar J. 2009. PMID: 19818172 Free PMC article. Review.
Cited by
-
Population genomics in neglected malaria parasites.Front Microbiol. 2022 Sep 8;13:984394. doi: 10.3389/fmicb.2022.984394. eCollection 2022. Front Microbiol. 2022. PMID: 36160257 Free PMC article. Review.
-
Characterization of Plasmodium infections among inhabitants of rural areas in Gabon.Sci Rep. 2019 Jul 5;9(1):9784. doi: 10.1038/s41598-019-46194-9. Sci Rep. 2019. PMID: 31278305 Free PMC article.
-
Polymorphic markers for identification of parasite population in Plasmodium malariae.Malar J. 2020 Jan 28;19(1):48. doi: 10.1186/s12936-020-3122-2. Malar J. 2020. PMID: 31992308 Free PMC article.
-
Plasmodium malariae structure and genetic diversity in sub-Saharan Africa determined from microsatellite variants and linked SNPs in orthologues of antimalarial resistance genes.Sci Rep. 2022 Dec 19;12(1):21881. doi: 10.1038/s41598-022-26625-w. Sci Rep. 2022. PMID: 36536036 Free PMC article.
-
Specificity of the IgG antibody response to Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale MSP119 subunit proteins in multiplexed serologic assays.Malar J. 2018 Nov 9;17(1):417. doi: 10.1186/s12936-018-2566-0. Malar J. 2018. PMID: 30413163 Free PMC article.
References
-
- Molineaux L, Storey J, Cohen JE, Thomas A. A longitudinal study of human malaria in the West African Savanna in the absence of control measures: relationships between different Plasmodium species, in particular P. falciparum and P. malariae. Am J Trop Med Hyg. 1980;29:725–37. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

