Distinct sequences and post-translational modifications in cardiac atrial and ventricular myosin light chains revealed by top-down mass spectrometry
- PMID: 28427997
- PMCID: PMC5526110
- DOI: 10.1016/j.yjmcc.2017.04.002
Distinct sequences and post-translational modifications in cardiac atrial and ventricular myosin light chains revealed by top-down mass spectrometry
Abstract
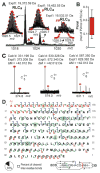
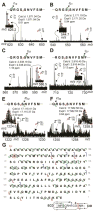
Myosin is the principal component of the thick filaments that, through interactions with the actin thin filaments, mediates force production during muscle contraction. Myosin is a hexamer, consisting of two heavy chains, each associated with an essential (ELC) and a regulatory (RLC) light chain, which bind the lever-arm of the heavy chain and play important modulatory roles in striated muscle contraction. Nevertheless, a comprehensive assessment of the sequences of the ELC and RLC isoforms, as well as their post-translational modifications, in the heart remains lacking. Herein, utilizing top-down high-resolution mass spectrometry (MS), we have comprehensively characterized the sequences and N-terminal modifications of the atrial and ventricular isoforms of the myosin light chains from human and swine hearts, as well as the sites of phosphorylation in the swine proteins. In addition to the correction of disparities in the database sequences of the swine proteins, we show for the first time that, whereas the ventricular isoforms of the ELC and RLC are methylated at their N-termini, which is consistent with previous studies, the atrial isoforms of the ELC and RLC from both human and swine are Nα-methylated and Nα-acetylated, respectively. Furthermore, top-down MS with electron capture dissociation enabled localization of the sites of phosphorylation in swine RLC isoforms from the ventricles and atria to Ser14 and Ser22, respectively. Collectively, these results provide new insights into the sequences and modifications of myosin light chain isoforms in the human and swine hearts, which will pave the way for a better understanding of their functional roles in cardiac physiology and pathophysiology.
Keywords: Acetylation; Methylation; Myosin light chain; Phosphorylation; Post-translational modification; Top-down mass spectrometry.
Copyright © 2017. Published by Elsevier Ltd.
Figures




Similar articles
-
Regulatory light chain phosphorylation augments length-dependent contraction in PTU-treated rats.J Gen Physiol. 2019 Jan 7;151(1):66-76. doi: 10.1085/jgp.201812158. Epub 2018 Dec 6. J Gen Physiol. 2019. PMID: 30523115 Free PMC article.
-
Effects of myosin light chain phosphorylation on length-dependent myosin kinetics in skinned rat myocardium.Arch Biochem Biophys. 2016 Jul 1;601:56-68. doi: 10.1016/j.abb.2015.12.014. Epub 2016 Jan 5. Arch Biochem Biophys. 2016. PMID: 26763941
-
Functional and Molecular Characterisation of Heart Failure Progression in Mice and the Role of Myosin Regulatory Light Chains in the Recovery of Cardiac Muscle Function.Int J Mol Sci. 2021 Dec 22;23(1):88. doi: 10.3390/ijms23010088. Int J Mol Sci. 2021. PMID: 35008512 Free PMC article.
-
Molecular mechanisms of cardiomyopathy phenotypes associated with myosin light chain mutations.J Muscle Res Cell Motil. 2015 Dec;36(6):433-45. doi: 10.1007/s10974-015-9423-3. Epub 2015 Sep 18. J Muscle Res Cell Motil. 2015. PMID: 26385864 Free PMC article. Review.
-
Role of myosin light chain phosphatase in cardiac physiology and pathophysiology.J Mol Cell Cardiol. 2016 Dec;101:35-43. doi: 10.1016/j.yjmcc.2016.10.004. Epub 2016 Oct 11. J Mol Cell Cardiol. 2016. PMID: 27742556 Free PMC article. Review.
Cited by
-
Study on the transcriptome for breast muscle of chickens and the function of key gene RAC2 on fibroblasts proliferation.BMC Genomics. 2021 Mar 6;22(1):157. doi: 10.1186/s12864-021-07453-0. BMC Genomics. 2021. PMID: 33676413 Free PMC article.
-
Fourier-transform ion cyclotron resonance mass spectrometry for characterizing proteoforms.Mass Spectrom Rev. 2022 Mar;41(2):158-177. doi: 10.1002/mas.21653. Epub 2020 Sep 7. Mass Spectrom Rev. 2022. PMID: 32894796 Free PMC article. Review.
-
HDAC Inhibition Regulates Cardiac Function by Increasing Myofilament Calcium Sensitivity and Decreasing Diastolic Tension.Pharmaceutics. 2022 Jul 21;14(7):1509. doi: 10.3390/pharmaceutics14071509. Pharmaceutics. 2022. PMID: 35890404 Free PMC article.
-
Modulating Nitric Oxide: Implications for Cytotoxicity and Cytoprotection.Antioxidants (Basel). 2024 Apr 23;13(5):504. doi: 10.3390/antiox13050504. Antioxidants (Basel). 2024. PMID: 38790609 Free PMC article. Review.
-
An Unbiased Proteomics Method to Assess the Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes.Circ Res. 2019 Nov 8;125(11):936-953. doi: 10.1161/CIRCRESAHA.119.315305. Epub 2019 Oct 1. Circ Res. 2019. PMID: 31573406 Free PMC article.
References
-
- Huxley HE. The mechanism of muscular contraction. Science. 1969;164(3886):1356–65. - PubMed
-
- Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233(5321):533–8. - PubMed
-
- Weeds AG, Lowey S. Substructure of the myosin molecule. II. The light chains of myosin. J Mol Biol. 1971;61(3):701–25. - PubMed
-
- Rayment I, Rypniewski WR, Schmidt-Bäse K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261(5117):50–8. - PubMed
-
- Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993;261(5117):58–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

