Comparison of methods for detecting asymptomatic malaria infections in the China-Myanmar border area
- PMID: 28427455
- PMCID: PMC5397696
- DOI: 10.1186/s12936-017-1813-0
Comparison of methods for detecting asymptomatic malaria infections in the China-Myanmar border area
Abstract
Background: Sensitive methods for detecting asymptomatic malaria infections are essential for identifying potential transmission reservoirs and obtaining an accurate assessment of malaria epidemiology in low-endemicity areas aiming to eliminate malaria. PCR techniques to detect parasite nucleic acids (DNA or RNA) are among the most commonly used molecular methods. However, most of these methods are of low throughput and cannot be used for large-scale molecular epidemiological studies. A recently developed capture and ligation probe-PCR (CLIP-PCR) is claimed to have the sensitivity of molecular techniques and the high throughput capacity needed for screening purposes. This study aimed to compare several molecular methods for detecting asymptomatic and submicroscopic Plasmodium infections in healthy residents of a malaria-hypoendemic region in Southeast Asia, where malaria elimination is in sight.
Method: This study compared three molecular detection methods side-by-side, namely nested PCR targeting the rRNA genes, nested RT-PCR to detect parasite rRNA, and CLIP-PCR to detect parasite rRNA in 1005 healthy individuals in northeastern Myanmar. For nested PCR and RT-PCR, parasite DNA and total RNA were extracted from ~100 µL of blood, whereas RNA used for CLIP-PCR was from a 3 mm disk of dried blood filter paper. The sensitivity and specificity of these methods were compared with those of conventional light microscopy. In addition, RT-PCR and quantitative RT-PCR (qRT-PCR) targeting the Pvs25 gene in Plasmodium vivax were used to assess gametocyte prevalence in the samples.
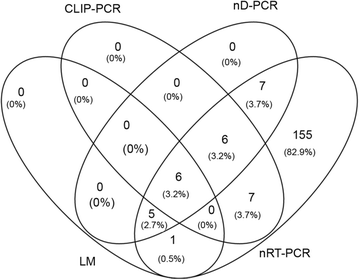
Results: Light microscopy detected Plasmodium infections in only 1.19% of the residents harbouring the parasites. CLIP-PCR had slightly better performance and detected Plasmodium infections in 1.89% of the population. Further improvement was achieved by nested PCR to detect parasite DNA, which detected P. vivax and Plasmodium falciparum infections in 2.39% of the residents. The nested RT-PCR targeting rRNA, however, detected as many as 187 (18.61%) individuals having Plasmodium infections with P. vivax being the predominant species (176 P. vivax, 5 P. falciparum and 6 P. falciparum/P. vivax mixed infections). Of the 210 Plasmodium-positive samples detected by all molecular methods, 115 were Pvs25-positive by qRT-PCR, indicating that a large proportion of asymptomatic individuals were gametocyte carriers.
Conclusion: Nested RT-PCR based on the detection of asexual-stage parasite rRNA was the most sensitive, with a more than sixfold higher sensitivity than the other two molecular methods of parasite detection. CLIP-PCR has an increased throughput, but its sensitivity in this study was much lower than those of other molecular methods, which may be partially due to the smaller amount of RNA input used.
Keywords: Asymptomatic; Capture and ligation probe-PCR; Light microscopy; Malaria; Nested PCR with DNA; Nested RT-PCR; Sensitivity; Specificity.
Figures
Similar articles
-
Plasmodium malariae and Plasmodium ovale infections in the China-Myanmar border area.Malar J. 2016 Nov 15;15(1):557. doi: 10.1186/s12936-016-1605-y. Malar J. 2016. PMID: 27846879 Free PMC article.
-
Comparison of detection methods to estimate asexual Plasmodium falciparum parasite prevalence and gametocyte carriage in a community survey in Tanzania.Malar J. 2014 Nov 18;13:433. doi: 10.1186/1475-2875-13-433. Malar J. 2014. PMID: 25404207 Free PMC article.
-
Geographical heterogeneity in prevalence of subclinical malaria infections at sentinel endemic sites of Myanmar.Parasit Vectors. 2019 Feb 18;12(1):83. doi: 10.1186/s13071-019-3330-1. Parasit Vectors. 2019. PMID: 30777127 Free PMC article.
-
Ultrasensitive Diagnostics for Low-Density Asymptomatic Plasmodium falciparum Infections in Low-Transmission Settings.J Clin Microbiol. 2021 Mar 19;59(4):e01508-20. doi: 10.1128/JCM.01508-20. Print 2021 Mar 19. J Clin Microbiol. 2021. PMID: 33148707 Free PMC article. Review.
-
Plasmodium vivax molecular diagnostics in community surveys: pitfalls and solutions.Malar J. 2018 Jan 30;17(1):55. doi: 10.1186/s12936-018-2201-0. Malar J. 2018. PMID: 29378609 Free PMC article. Review.
Cited by
-
Prevalence of Asymptomatic Malaria among Children in the Tamale Metropolis: How Does the PfHRP2 CareStart™ RDT Perform against Microscopy?J Trop Med. 2019 Dec 21;2019:6457628. doi: 10.1155/2019/6457628. eCollection 2019. J Trop Med. 2019. PMID: 31933652 Free PMC article.
-
The detection of cryptic Plasmodium infection among villagers in Attapeu province, Lao PDR.PLoS Negl Trop Dis. 2017 Dec 20;11(12):e0006148. doi: 10.1371/journal.pntd.0006148. eCollection 2017 Dec. PLoS Negl Trop Dis. 2017. PMID: 29261647 Free PMC article.
-
Efficacy and Safety of a Naphthoquine-Azithromycin Coformulation for Malaria Prophylaxis in Southeast Asia: A Phase 3, Double-blind, Randomized, Placebo-controlled Trial.Clin Infect Dis. 2021 Oct 5;73(7):e2470-e2476. doi: 10.1093/cid/ciaa1018. Clin Infect Dis. 2021. PMID: 32687174 Free PMC article. Clinical Trial.
-
Malaria burden and treatment targets in Kachin Special Region II, Myanmar from 2008 to 2016: A retrospective analysis.PLoS One. 2018 Apr 3;13(4):e0195032. doi: 10.1371/journal.pone.0195032. eCollection 2018. PLoS One. 2018. PMID: 29614088 Free PMC article.
-
Genetic Variations Associated with Drug Resistance Markers in Asymptomatic Plasmodium falciparum Infections in Myanmar.Genes (Basel). 2019 Sep 9;10(9):692. doi: 10.3390/genes10090692. Genes (Basel). 2019. PMID: 31505774 Free PMC article.
References
-
- WHO . World malaria report 2016. Geneva: World Health Organization; 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

