Down-regulation of long non-coding RNA RP11-708H21.4 is associated with poor prognosis for colorectal cancer and promotes tumorigenesis through regulating AKT/mTOR pathway
- PMID: 28427191
- PMCID: PMC5438619
- DOI: 10.18632/oncotarget.15846
Down-regulation of long non-coding RNA RP11-708H21.4 is associated with poor prognosis for colorectal cancer and promotes tumorigenesis through regulating AKT/mTOR pathway
Abstract
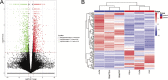
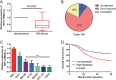
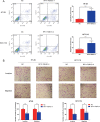
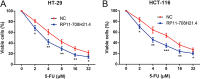
Long non-coding RNAs (lncRNAs) serve critical roles in cancer development and progression. Herein, through next generation RNA sequencing and experimental validations, we determined the expression status of RP11-708H21.4 in colorectal cancer (CRC) and explored its clinical significance and biological functions in CRC. Differentially expressed lncRNAs from CRC samples and corresponding normal mucosa tissues was screened through RNA sequencing, and RP11-708H21.4 was selected for further experimental validation. The expression levels of RP11-708H21.4 in CRC tissues and cell lines were determined using qRT-PCR. Also, the relationship between the clinicopathological features and RP11-708H21.4 expression was analyzed. Cell viability was examined by CCK-8 and colony assays; cell migration and invasion were detected by transwell assays; cell cycle and cell apoptosis were analyzed by flow cytometry. The chemosensitivity of CRC cells to 5-Fluorouracil (5-FU) was also determined using CCK-8 assay. CRC xenograft tumor models were established to determine the biological functions of RP11-708H21.4 in vivo. Levels of cell cycle-related proteins and AKT/mTOR pathway-related proteins were detected by western blot assay. RP11-708H21.4 expression was aberrantly decreased in CRC, and its expression was closely associated with aggressive clinicopathologic features and unfavorable prognosis of CRC patients. Overexpressed RP11-708H21.4 suppresses CRC cell proliferation through inducing G1 arrest. Moreover, up-regulation of RP11-708H21.4 inhibits cell migration and invasion, causes cell apoptosis, and enhances 5-FU sensitivity of CRC cells. Finally, increased RP11-708H21.4 expression blocked AKT/mTOR pathway, and repressed in vivo CRC xenograft tumor growth. The results indicated that RP11-708H21.4 might have potential roles as a biomarker and a therapeutic target for CRC.
Keywords: RNA sequencing; RP11-708H21.4; colorectal cancer; long noncoding RNA; tumorigenesis.
Conflict of interest statement
None.
Figures


Similar articles
-
Diagnostic and prognostic potential of tissue and circulating long non-coding RNAs in colorectal tumors.World J Gastroenterol. 2019 Sep 14;25(34):5026-5048. doi: 10.3748/wjg.v25.i34.5026. World J Gastroenterol. 2019. PMID: 31558855 Free PMC article. Review.
-
Long noncoding RNA RP11-757G1.5 sponges miR-139-5p and upregulates YAP1 thereby promoting the proliferation and liver, spleen metastasis of colorectal cancer.J Exp Clin Cancer Res. 2020 Oct 6;39(1):207. doi: 10.1186/s13046-020-01717-5. J Exp Clin Cancer Res. 2020. PMID: 33023613 Free PMC article.
-
Downregulation of LncRNA-RP11-317J10.2 promotes cell proliferation and invasion and predicts poor prognosis in colorectal cancer.Scand J Gastroenterol. 2018 Jan;53(1):38-45. doi: 10.1080/00365521.2017.1392597. Epub 2017 Oct 26. Scand J Gastroenterol. 2018. PMID: 29073791
-
Super-enhancer-associated long noncoding RNA RP11-569A11.1 inhibited cell progression and metastasis by regulating IFIT2 in colorectal cancer.J Clin Lab Anal. 2021 Jun;35(6):e23780. doi: 10.1002/jcla.23780. Epub 2021 May 4. J Clin Lab Anal. 2021. PMID: 33942366 Free PMC article.
-
Non-coding RNAs as Biomarkers for Survival in Colorectal Cancer Patients.Curr Aging Sci. 2024;17(1):5-15. doi: 10.2174/1874609816666230202101054. Curr Aging Sci. 2024. PMID: 36733201 Review.
Cited by
-
LINC01272/miR-876/ITGB2 axis facilitates the metastasis of colorectal cancer via epithelial-mesenchymal transition.J Cancer. 2021 May 5;12(13):3909-3919. doi: 10.7150/jca.55666. eCollection 2021. J Cancer. 2021. PMID: 34093798 Free PMC article.
-
MEG3: an Oncogenic Long Non-coding RNA in Different Cancers.Pathol Oncol Res. 2019 Jul;25(3):859-874. doi: 10.1007/s12253-019-00614-3. Epub 2019 Feb 21. Pathol Oncol Res. 2019. PMID: 30793226 Review.
-
Diagnostic and prognostic potential of tissue and circulating long non-coding RNAs in colorectal tumors.World J Gastroenterol. 2019 Sep 14;25(34):5026-5048. doi: 10.3748/wjg.v25.i34.5026. World J Gastroenterol. 2019. PMID: 31558855 Free PMC article. Review.
-
Interaction of lncRNAs with mTOR in colorectal cancer: a systematic review.BMC Cancer. 2023 Jun 6;23(1):512. doi: 10.1186/s12885-023-11008-9. BMC Cancer. 2023. PMID: 37280524 Free PMC article.
-
The interplay between m6A RNA methylation and noncoding RNA in cancer.J Hematol Oncol. 2019 Nov 22;12(1):121. doi: 10.1186/s13045-019-0805-7. J Hematol Oncol. 2019. PMID: 31757221 Free PMC article. Review.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. - PubMed
-
- Li HZ, Mao WM, Wang XH, Yu CD, Du LB. Incidence and mortality of cancer in Zhejiang province in 2009. Zhonghua yu fang yi xue za zhi. 2013;47:592–6. [Article in Chinese] - PubMed
-
- Liu F, Dear K, Huang L, Liu L, Shi Y, Nie S, Liu Y, Lu Y, Xiang H. Association between microRNA-27a rs895819 polymorphism and risk of colorectal cancer: A meta-analysis. Cancer Genet. 2016;209:388–94. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

