Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma
- PMID: 28424871
- PMCID: PMC5522523
- DOI: 10.1007/s00384-017-2800-1
Final analysis of the randomised PEAK trial: overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma
Abstract
Purpose: To report planned final overall (OS) and progression-free survival (PFS) analyses from the phase II PEAK trial (NCT00819780).
Methods: Patients with previously untreated, KRAS exon 2 wild-type (WT) metastatic colorectal cancer (mCRC) were randomised to mFOLFOX6 plus panitumumab or bevacizumab. The primary endpoint was PFS; secondary endpoints included OS, objective response rate, duration of response (DoR), time to response, resection and safety. Treatment effect by tumour RAS status was a prespecified objective. Exploratory analyses included early tumour shrinkage (ETS) and depth of response (DpR).
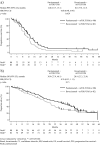
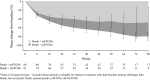
Results: One hundred seventy patients had RAS WT and 156 had RAS WT/BRAF WT mCRC. Median PFS was longer for panitumumab versus bevacizumab in the RAS WT (12.8 vs 10.1 months; hazard ratio (HR) = 0.68 [95% confidence intervals (CI) = 0.48-0.96]; p = 0.029) and RAS WT/BRAF WT (13.1 vs 10.1 months; HR = 0.61 [95% CI = 0.42-0.88]; p = 0.0075) populations. Median OS (68% OS events) for panitumumab versus bevacizumab was 36.9 versus 28.9 months (HR = 0.76 [95% CI = 0.53-1.11]; p = 0.15) and 41.3 versus 28.9 months (HR = 0.70 [95% CI = 0.48-1.04]; p = 0.08), in the RAS WT and RAS WT/BRAF WT populations, respectively. Median DoR (11.4 vs 9.0 months; HR = 0.59 [95% CI = 0.39-0.88]; p = 0.011) and DpR (65.0 vs 46.3%; p = 0.0018) were improved in the panitumumab group. More panitumumab patients experienced ≥30% ETS at week 8 (64 vs 45%; p = 0.052); ETS was associated with improved PFS/OS. No new safety signals occurred.
Conclusions: First-line panitumumab + mFOLFOX6 increases PFS versus bevacizumab + mFOLFOX6 in patients with RAS WT mCRC.
Keywords: Bevacizumab; First-line; Metastatic colorectal cancer; Overall survival; Panitumumab.
Conflict of interest statement
Conflict of interest
FR has acted on advisory boards and received research funding from Amgen, during the course of this study. He has also acted on advisory boards or received research funding from Bayer, Lilly, Merck-Serono, Merck Sharp and Dohme, Roche, Sanofi, and Servier, outside of the submitted work. MK reports personal fees from Amgen and Roche, outside the submitted work. JRH reports personal fees from Amgen and Roche, outside the submitted work. IS has acted on advisory boards/received honoraria from Amgen, Roche, Merck-Serono, Sanofi, Bayer, Ipsen and Novartis. FF has acted on advisory boards/received honoraria from Novartis and Nutricia, and received research grants from Amgen, outside the submitted work. GF reports grants and personal fees from Amgen, during the course of the study. JLC reports research grants from Amgen, outside the submitted work. XG is an employee of Amgen Inc. GD is an employee of Amgen (Europe) GmbH. LSS has acted as a consultant and on a speaker’s bureau for Amgen, outside the submitted work.
Statement of human rights
PEAK (NCT00819780) was conducted in compliance with the Declaration of Helsinki. The study protocol was approved by an independent ethics committee at each participating study centre.
Informed consent
All patients provided informed consent before any study procedures were performed.
Figures
Similar articles
-
PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer.J Clin Oncol. 2014 Jul 20;32(21):2240-7. doi: 10.1200/JCO.2013.53.2473. Epub 2014 Mar 31. J Clin Oncol. 2014. PMID: 24687833 Clinical Trial.
-
Exploratory analyses assessing the impact of early tumour shrinkage and depth of response on survival outcomes in patients with RAS wild-type metastatic colorectal cancer receiving treatment in three randomised panitumumab trials.J Cancer Res Clin Oncol. 2018 Feb;144(2):321-335. doi: 10.1007/s00432-017-2534-z. Epub 2017 Oct 28. J Cancer Res Clin Oncol. 2018. PMID: 29080924 Free PMC article. Clinical Trial.
-
Panitumumab vs Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival Among Patients With RAS Wild-type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial.JAMA. 2023 Apr 18;329(15):1271-1282. doi: 10.1001/jama.2023.4428. JAMA. 2023. PMID: 37071094 Free PMC article. Clinical Trial.
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
-
A meta-analysis of efficacy and safety data from head-to-head first-line trials of epidermal growth factor receptor inhibitors versus bevacizumab in adult patients with RAS wild-type metastatic colorectal cancer by sidedness.Eur J Cancer. 2024 May;202:113975. doi: 10.1016/j.ejca.2024.113975. Epub 2024 Mar 1. Eur J Cancer. 2024. PMID: 38442645 Review.
Cited by
-
Safety of first-line systemic therapy in patients with metastatic colorectal cancer: a network meta-analysis of randomized controlled trials.BMC Cancer. 2024 Jul 24;24(1):893. doi: 10.1186/s12885-024-12662-3. BMC Cancer. 2024. PMID: 39048944 Free PMC article.
-
Limited Liver or Lung Colorectal Cancer Metastases. Systemic Treatment, Surgery, Ablation or SBRT.J Clin Med. 2021 May 14;10(10):2131. doi: 10.3390/jcm10102131. J Clin Med. 2021. PMID: 34069240 Free PMC article. Review.
-
Optimizing biologic sequencing in metastatic colorectal cancer: first line and beyond.Curr Oncol. 2019 Nov;26(Suppl 1):S33-S42. doi: 10.3747/co.26.5589. Epub 2019 Nov 1. Curr Oncol. 2019. PMID: 31819708 Free PMC article. Review.
-
Targeted Therapies in Colorectal Cancer: Recent Advances in Biomarkers, Landmark Trials, and Future Perspectives.Cancers (Basel). 2023 Jun 1;15(11):3023. doi: 10.3390/cancers15113023. Cancers (Basel). 2023. PMID: 37296986 Free PMC article. Review.
-
Evaluation of histopathological heterogeneity of colorectal cancer liver metastasis sites after preoperative chemotherapy.Mol Clin Oncol. 2022 Mar;16(3):61. doi: 10.3892/mco.2022.2494. Epub 2022 Jan 13. Mol Clin Oncol. 2022. PMID: 35127086 Free PMC article.
References
-
- National Comprehensive Cancer Network® (2015) Clinical practice guidelines in oncology (NCCN Guidelines®): colon cancer version 1.2015. Available at: www.nccn.org/. Access date 15 March 2017 - PubMed
-
- Koike J, Ushigome M, Funahashi K, Shiokawa H, Kaneko T, Arai K, Matsuda S, Kagami S, Suzuki T, Kurihara A, Shimada H, Kaneko H. Significance of KRAS mutation in patients receiving mFOLFOX6 with or without bevacizumab for metastatic colorectal cancer. Hepato-Gastroenterology. 2014;61:2222–2226. - PubMed
-
- Price TJ, Bruhn MA, Lee CK, Hardingham JE, Townsend AR, Mann KP, Simes J, Weickhardt A, Wrin JW, Wilson K, Gebski V, Van HG, Robinson B, Cunningham D, Tebbutt NC. Correlation of extended RAS and PIK3CA gene mutation status with outcomes from the phase III AGITG MAX STUDY involving capecitabine alone or in combination with bevacizumab plus or minus mitomycin C in advanced colorectal cancer. Br J Cancer. 2015;112:963–970. doi: 10.1038/bjc.2015.37. - DOI - PMC - PubMed
-
- European Medicines Agency (2015) Vectibix® (panitumumab) Summary of Product Characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info.... Access date 15 March 2017
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

