CD103+ CD8 T Cells in the Toxoplasma-Infected Brain Exhibit a Tissue-Resident Memory Transcriptional Profile
- PMID: 28424687
- PMCID: PMC5372813
- DOI: 10.3389/fimmu.2017.00335
CD103+ CD8 T Cells in the Toxoplasma-Infected Brain Exhibit a Tissue-Resident Memory Transcriptional Profile
Abstract
During chronic infection, memory T cells acquire a unique phenotype and become dependent on different survival signals than those needed for memory T cells generated during an acute infection. The distinction between the role of effector and memory T cells in an environment of persistent antigen remains unclear. Here, in the context of chronic Toxoplasma gondii infection, we demonstrate that a population of CD8 T cells exhibiting a tissue-resident memory (TRM) phenotype accumulates within the brain. We show that this population is distributed throughout the brain in both parenchymal and extraparenchymal spaces. Furthermore, this population is transcriptionally distinct and exhibits a transcriptional signature consistent with the TRM observed in acute viral infections. Finally, we establish that the CD103+ TRM population has an intrinsic capacity to produce both IFN-γ and TNF-α, cytokines critical for parasite control within the central nervous system (CNS). The contribution of this population to pro-inflammatory cytokine production suggests an important role for TRM in protective and ongoing immune responses in the infected CNS. Accession number: GSE95105.
Keywords: CD103; CD8+ T cell memory; Toxoplasma gondii; chronic infection; neuroimmunology; tissue-resident memory cells.
Figures

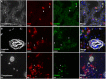
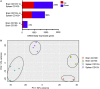

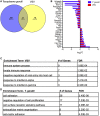
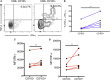
Similar articles
-
Functional Heterogeneity of CD4+ Tumor-Infiltrating Lymphocytes With a Resident Memory Phenotype in NSCLC.Front Immunol. 2018 Nov 16;9:2654. doi: 10.3389/fimmu.2018.02654. eCollection 2018. Front Immunol. 2018. PMID: 30505306 Free PMC article.
-
Attenuated Oral Typhoid Vaccine Ty21a Elicits Lamina Propria and Intra-Epithelial Lymphocyte Tissue-Resident Effector Memory CD8 T Responses in the Human Terminal Ileum.Front Immunol. 2019 Mar 14;10:424. doi: 10.3389/fimmu.2019.00424. eCollection 2019. Front Immunol. 2019. PMID: 30923521 Free PMC article. Clinical Trial.
-
Oral typhoid vaccine Ty21a elicits antigen-specific resident memory CD4+ T cells in the human terminal ileum lamina propria and epithelial compartments.J Transl Med. 2020 Feb 25;18(1):102. doi: 10.1186/s12967-020-02263-6. J Transl Med. 2020. PMID: 32098623 Free PMC article.
-
The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin.Front Immunol. 2018 Aug 15;9:1904. doi: 10.3389/fimmu.2018.01904. eCollection 2018. Front Immunol. 2018. PMID: 30158938 Free PMC article. Review.
-
Tissue-Resident Memory CD8+ T Cells: From Phenotype to Function.Front Immunol. 2018 Mar 26;9:515. doi: 10.3389/fimmu.2018.00515. eCollection 2018. Front Immunol. 2018. PMID: 29632527 Free PMC article. Review.
Cited by
-
Tissue-Resident T Cells in Chronic Relapsing-Remitting Intestinal Disorders.Cells. 2021 Jul 25;10(8):1882. doi: 10.3390/cells10081882. Cells. 2021. PMID: 34440651 Free PMC article. Review.
-
Brain-Resident T Cells Following Viral Infection.Viral Immunol. 2019 Jan/Feb;32(1):48-54. doi: 10.1089/vim.2018.0084. Epub 2018 Sep 18. Viral Immunol. 2019. PMID: 30230418 Free PMC article. Review.
-
T Cell Receptor-Major Histocompatibility Complex Interaction Strength Defines Trafficking and CD103+ Memory Status of CD8 T Cells in the Brain.Front Immunol. 2018 Jun 5;9:1290. doi: 10.3389/fimmu.2018.01290. eCollection 2018. Front Immunol. 2018. PMID: 29922298 Free PMC article.
-
Niches for the Long-Term Maintenance of Tissue-Resident Memory T Cells.Front Immunol. 2018 May 31;9:1214. doi: 10.3389/fimmu.2018.01214. eCollection 2018. Front Immunol. 2018. PMID: 29904388 Free PMC article. Review.
-
Systemic Listeria monocytogenes infection in aged mice induces long-term neuroinflammation: the role of miR-155.Immun Ageing. 2022 May 25;19(1):25. doi: 10.1186/s12979-022-00281-0. Immun Ageing. 2022. PMID: 35614490 Free PMC article.
References
-
- Kivisakk P, Trebst C, Liu Z, Tucky BH, Sorensen TL, Rudick RA, et al. T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin Exp Immunol (2002) 129(3):510–8.10.1046/j.1365-2249.2002.01947.x - DOI - PMC - PubMed
-
- Wilson DC, Grotenbreg GM, Liu K, Zhao Y, Frickel EM, Gubbels MJ, et al. Differential regulation of effector- and central-memory responses to Toxoplasma gondii infection by IL-12 revealed by tracking of Tgd057-specific CD8+ T cells. PLoS Pathog (2010) 6(3):e1000815.10.1371/journal.ppat.1000815 - DOI - PMC - PubMed
-
- Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol (1992) 149(1):175–80. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

