APP Protein Family Signaling at the Synapse: Insights from Intracellular APP-Binding Proteins
- PMID: 28424586
- PMCID: PMC5371672
- DOI: 10.3389/fnmol.2017.00087
APP Protein Family Signaling at the Synapse: Insights from Intracellular APP-Binding Proteins
Abstract
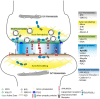
Understanding the molecular mechanisms underlying amyloid precursor protein family (APP/APP-like proteins, APLP) function in the nervous system can be achieved by studying the APP/APLP interactome. In this review article, we focused on intracellular APP interacting proteins that bind the YENPTY internalization motif located in the last 15 amino acids of the C-terminal region. These proteins, which include X11/Munc-18-interacting proteins (Mints) and FE65/FE65Ls, represent APP cytosolic binding partners exhibiting different neuronal functions. A comparison of FE65 and APP family member mutant mice revealed a shared function for APP/FE65 protein family members in neurogenesis and neuronal positioning. Accumulating evidence also supports a role for membrane-associated APP/APLP proteins in synapse formation and function. Therefore, it is tempting to speculate that APP/APLP C-terminal interacting proteins transmit APP/APLP-dependent signals at the synapse. Herein, we compare our current knowledge of the synaptic phenotypes of APP/APLP mutant mice with those of mice lacking different APP/APLP interaction partners and discuss the possible downstream effects of APP-dependent FE65/FE65L or X11/Mint signaling on synaptic vesicle release, synaptic morphology and function. Given that the role of X11/Mint proteins at the synapse is well-established, we propose a model highlighting the role of FE65 protein family members for transduction of APP/APLP physiological function at the synapse.
Keywords: FE65; FE65L1; X11/Mint proteins; amyloid precursor protein (APP); synaptic signaling.
Figures
Similar articles
-
Fe65L2: a new member of the Fe65 protein family interacting with the intracellular domain of the Alzheimer's beta-amyloid precursor protein.Biochem J. 1998 Feb 15;330 ( Pt 1)(Pt 1):513-9. doi: 10.1042/bj3300513. Biochem J. 1998. PMID: 9461550 Free PMC article.
-
APLP1 Is a Synaptic Cell Adhesion Molecule, Supporting Maintenance of Dendritic Spines and Basal Synaptic Transmission.J Neurosci. 2017 May 24;37(21):5345-5365. doi: 10.1523/JNEUROSCI.1875-16.2017. Epub 2017 Apr 27. J Neurosci. 2017. PMID: 28450540 Free PMC article.
-
FE65 and FE65L1 share common synaptic functions and genetically interact with the APP family in neuromuscular junction formation.Sci Rep. 2016 May 11;6:25652. doi: 10.1038/srep25652. Sci Rep. 2016. PMID: 27734846 Free PMC article.
-
The role of APP and APLP for synaptic transmission, plasticity, and network function: lessons from genetic mouse models.Exp Brain Res. 2012 Apr;217(3-4):435-40. doi: 10.1007/s00221-011-2894-6. Epub 2011 Oct 18. Exp Brain Res. 2012. PMID: 22006270 Review.
-
Functional Roles of the Interaction of APP and Lipoprotein Receptors.Front Mol Neurosci. 2017 Mar 1;10:54. doi: 10.3389/fnmol.2017.00054. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28298885 Free PMC article. Review.
Cited by
-
Amyloid Precursor Protein: A Regulatory Hub in Alzheimer's Disease.Aging Dis. 2024 Feb 1;15(1):201-225. doi: 10.14336/AD.2023.0308. Aging Dis. 2024. PMID: 37307834 Free PMC article. Review.
-
Tandem Mass Spectrometry as Strategy for the Selective Identification and Quantification of the Amyloid Precursor Protein Tyr682 Residue Phosphorylation Status in Human Blood Mononuclear Cells.Biomolecules. 2021 Aug 31;11(9):1297. doi: 10.3390/biom11091297. Biomolecules. 2021. PMID: 34572510 Free PMC article.
-
G-quadruplexes and associated proteins in aging and Alzheimer's disease.Front Aging. 2023 Jun 1;4:1164057. doi: 10.3389/fragi.2023.1164057. eCollection 2023. Front Aging. 2023. PMID: 37323535 Free PMC article. Review.
-
Amyloid β and Amyloid Precursor Protein Synergistically Suppress Large-Conductance Calcium-Activated Potassium Channel in Cortical Neurons.Front Aging Neurosci. 2021 Jun 3;13:660319. doi: 10.3389/fnagi.2021.660319. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34149396 Free PMC article.
-
Direct evidence of amyloid precursor-like protein 1 trans interactions in cell-cell adhesion platforms investigated via fluorescence fluctuation spectroscopy.Mol Biol Cell. 2017 Dec 1;28(25):3609-3620. doi: 10.1091/mbc.E17-07-0459. Epub 2017 Oct 11. Mol Biol Cell. 2017. PMID: 29021345 Free PMC article.
References
-
- Araki Y., Tomita S., Yamaguchi H., Miyagi N., Sumioka A., Kirino Y., et al. . (2003). Novel cadherin-related membrane proteins, Alcadeins, enhance the X11-like protein-mediated stabilization of amyloid β-protein precursor metabolism. J. Biol. Chem. 278, 49448–49458. 10.1074/jbc.m306024200 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

