The SNAIL/miR-128 axis regulated growth, invasion, metastasis, and epithelial-to-mesenchymal transition of gastric cancer
- PMID: 28424413
- PMCID: PMC5503613
- DOI: 10.18632/oncotarget.16849
The SNAIL/miR-128 axis regulated growth, invasion, metastasis, and epithelial-to-mesenchymal transition of gastric cancer
Abstract
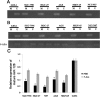
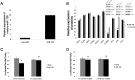
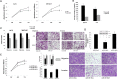
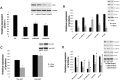
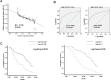
miR-128 is expressed in various tumors, but its expression and function in gastric cancer have not been defined. Thus, the goal of this study was to characterize miR-128 in gastric cancer. We found first that miR-128 is down-regulated in gastric cancer cell lines and tissues, and this dysregulation is correlated with DNA methylation and the transcription factor SNAIL. Using prediction tools, western blotting, and luciferase reporter assays, we found that Bmi-1 was the direct target of miR-128. Additionally, overexpression of miR-128 inhibited gastric cancer cell migration, invasion, and proliferation by targeting Bmi-1 in vitro and in vivo. We also documented, with receiver operating characteristic curves and Kaplan-Meier survival analysis, that miR-128 and Bmi-1 may be useful markers for diagnosing and estimating the prognosis of gastric cancer patients. As the epithelial-to-mesenchymal transition is an important mechanism associated with cancer invasion and metastasis, we inferred that miR-128 could regulate this mechanism in gastric cancer. In fact, we found that miR-128 could reverse epithelial-to-mesenchymal transition induced by Bmi-1 via the PI3K/AKT pathway. Because SNAIL also acts as a mesenchymal marker, our findings identified a novel positive feedback loop in which the transcription factor SNAIL curbs the expression of miR-128, and then down-regulated miR-128 promotes the expression of Bmi-1; finally, overexpression of Bmi-1 drives the epithelial-to-mesenchymal transition process via the PI3K/AKT pathway, and the expression of SNAIL is up-regulated.
Keywords: DNA methylation; SNAIL; gastric cancer; miR-128; transcription factor.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

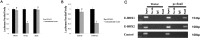

Similar articles
-
microRNA-33a prevents epithelial-mesenchymal transition, invasion, and metastasis of gastric cancer cells through the Snail/Slug pathway.Am J Physiol Gastrointest Liver Physiol. 2019 Aug 1;317(2):G147-G160. doi: 10.1152/ajpgi.00284.2018. Epub 2019 Apr 3. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30943047
-
The miR-506-Induced Epithelial-Mesenchymal Transition is Involved in Poor Prognosis for Patients with Gastric Cancer.Ann Surg Oncol. 2015 Dec;22 Suppl 3:S1436-43. doi: 10.1245/s10434-015-4418-2. Epub 2015 Feb 24. Ann Surg Oncol. 2015. PMID: 25707493
-
A-kinase-interacting protein 1 facilitates growth and metastasis of gastric cancer cells via Slug-induced epithelial-mesenchymal transition.J Cell Mol Med. 2019 Jun;23(6):4434-4442. doi: 10.1111/jcmm.14339. Epub 2019 Apr 24. J Cell Mol Med. 2019. PMID: 31020809 Free PMC article.
-
MiR-146a functions as a small silent player in gastric cancer.Biomed Pharmacother. 2017 Dec;96:238-245. doi: 10.1016/j.biopha.2017.09.138. Epub 2017 Oct 6. Biomed Pharmacother. 2017. PMID: 28987948 Review.
-
Epithelial mesenchymal transition Transcription Factor (TF): The structure, function and microRNA feedback loop.Gene. 2018 Oct 20;674:115-120. doi: 10.1016/j.gene.2018.06.049. Epub 2018 Jun 21. Gene. 2018. PMID: 29936265 Review.
Cited by
-
Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer.Oncogene. 2018 Sep;37(36):4903-4920. doi: 10.1038/s41388-018-0341-x. Epub 2018 May 23. Oncogene. 2018. PMID: 29795331 Free PMC article. Review.
-
PAICS, a De Novo Purine Biosynthetic Enzyme, Is Overexpressed in Pancreatic Cancer and Is Involved in Its Progression.Transl Oncol. 2020 Jul;13(7):100776. doi: 10.1016/j.tranon.2020.100776. Epub 2020 May 15. Transl Oncol. 2020. PMID: 32422575 Free PMC article.
-
MicroRNA (miR) dysregulation during Helicobacter pylori-induced gastric inflammation and cancer development: critical importance of miR-155.Oncotarget. 2020 Mar 10;11(10):894-904. doi: 10.18632/oncotarget.27520. eCollection 2020 Mar 10. Oncotarget. 2020. PMID: 32206186 Free PMC article.
-
MicroRNA-216a inhibits the metastasis of gastric cancer cells by targeting JAK2/STAT3-mediated EMT process.Oncotarget. 2017 Oct 4;8(51):88870-88881. doi: 10.18632/oncotarget.21488. eCollection 2017 Oct 24. Oncotarget. 2017. PMID: 29179483 Free PMC article.
-
Epithelial-Mesenchymal Transition: Role in Cancer Progression and the Perspectives of Antitumor Treatment.Acta Naturae. 2020 Jul-Sep;12(3):4-23. doi: 10.32607/actanaturae.11010. Acta Naturae. 2020. PMID: 33173593 Free PMC article.
References
-
- Lau M, Le A El-Serag HB. Noncardia gastric adenocarcinoma remains an important and deadly cancer in the United States: secular trends in incidence and survival. Am J Gastroenterol. 2006;101:2485–2492. - PubMed
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. - PubMed
-
- Shin CM, Kim N, Lee HS, Lee DH, Kim JS, Jung HC, Song IS. Intrafamilial aggregation of gastric cancer: a comprehensive approach including environmental factors, Helicobacter pylori virulence, and genetic susceptibility. Eur J Gastroenterol Hepatol. 2011;23:411–417. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

