Design, synthesis and structure-activity relationship study of wollamide B; a new potential anti TB agent
- PMID: 28423019
- PMCID: PMC5397059
- DOI: 10.1371/journal.pone.0176088
Design, synthesis and structure-activity relationship study of wollamide B; a new potential anti TB agent
Abstract
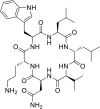
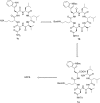
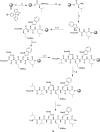
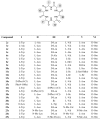
Wollamide B is a cationic antimycobacterial cyclohexapeptide that exhibits activity against Mycobacterium bovis (M. bovis) (IC50 of 3.1 μM). Aiming to define its structural activity relationship (SAR), optimizing potency and pharmacokinetic properties, libraries of analogues were synthesized following a standard Fmoc-based solid phase peptide synthesis approach. The antimycobacterial activities of wollamide B and all the synthesized analogues were tested against Mycobacterium tuberculosis (Mtb) H37Rv. Parallely, in vitro drug metabolism and pharmacokinetic (ADME) profiling was done for the synthesized compounds to evaluate their drug likeness. Among the 25 synthesized wollamides five of them showed potent activities with MICs ≤ 3.1 μM and found to be nontoxic against human HepG2 cells up to 100 μM. The results of the in vitro ADME profiling revealed the remarkable plasma stability and very good aqueous solubility of the class in general while the metabolic stability was found to be moderate to low. Of particular note, compounds 7c (MIC = 1.1 μM) and 13c (0.6 μM) that exhibited good balance of antimycobacterial activity vs. optimal pharmacokinetic properties could be used as a new lead for further development.
Conflict of interest statement
Figures
Similar articles
-
Structure-Activity Relationships of Wollamide Cyclic Hexapeptides with Activity against Drug-Resistant and Intracellular Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2019 Feb 26;63(3):e01773-18. doi: 10.1128/AAC.01773-18. Print 2019 Mar. Antimicrob Agents Chemother. 2019. PMID: 30602509 Free PMC article.
-
An efficient synthetic route for preparation of antimycobacterial wollamides and evaluation of their in vitro and in vivo efficacy.Bioorg Med Chem Lett. 2018 Sep 15;28(17):2899-2905. doi: 10.1016/j.bmcl.2018.07.021. Epub 2018 Jul 19. Bioorg Med Chem Lett. 2018. PMID: 30031620
-
Solid-Phase Synthesis and Antibacterial Activity of Cyclohexapeptide Wollamide B Analogs.ACS Comb Sci. 2018 Mar 12;20(3):172-185. doi: 10.1021/acscombsci.7b00189. Epub 2018 Feb 20. ACS Comb Sci. 2018. PMID: 29431987
-
Solid-Phase Synthesis of Wollamide Cyclohexapeptide Analogs.Methods Mol Biol. 2020;2103:175-187. doi: 10.1007/978-1-0716-0227-0_11. Methods Mol Biol. 2020. PMID: 31879925
-
Nitrofurans as novel anti-tuberculosis agents: identification, development and evaluation.Curr Top Med Chem. 2007;7(5):509-26. doi: 10.2174/156802607780059772. Curr Top Med Chem. 2007. PMID: 17346196 Review.
Cited by
-
Toward the Synthesis and Improved Biopotential of an N-methylated Analog of a Proline-Rich Cyclic Tetrapeptide from Marine Bacteria.Mar Drugs. 2018 Aug 30;16(9):305. doi: 10.3390/md16090305. Mar Drugs. 2018. PMID: 30200225 Free PMC article.
-
Bifurcation drives the evolution of assembly-line biosynthesis.Nat Commun. 2022 Jun 17;13(1):3498. doi: 10.1038/s41467-022-30950-z. Nat Commun. 2022. PMID: 35715397 Free PMC article.
-
Structure-Activity Relationships of Wollamide Cyclic Hexapeptides with Activity against Drug-Resistant and Intracellular Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2019 Feb 26;63(3):e01773-18. doi: 10.1128/AAC.01773-18. Print 2019 Mar. Antimicrob Agents Chemother. 2019. PMID: 30602509 Free PMC article.
-
"Upcycling" known molecules and targets for drug-resistant TB.Front Cell Infect Microbiol. 2022 Oct 6;12:1029044. doi: 10.3389/fcimb.2022.1029044. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36275029 Free PMC article.
-
The Antitubercular Activities of Natural Products with Fused-Nitrogen-Containing Heterocycles.Pharmaceuticals (Basel). 2024 Feb 6;17(2):211. doi: 10.3390/ph17020211. Pharmaceuticals (Basel). 2024. PMID: 38399426 Free PMC article. Review.
References
-
- WHO. Global tuberculosis report 2015, 2015.
-
- Godebo A, Abiy H, Toma A. Recent Advances in the Development of Anti-tuberculosis Drugs Acting on Multidrug-Resistant Strains: A review. International Journal of Research in Pharmacy and Biosciences. 2015;2: 1–18. Available: http://www.ijrpb.org/pdf/v2-i5/1.pdf
-
- Hancock REW, Lehrer R. Cationic peptides: A new source of antibiotics. Trends in Biotechnology. 1998;16: 82–88. - PubMed
-
- Hancock REW, Sahl H. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnology. 2006;24: 1551–1557. doi: 10.1038/nbt1267 - DOI - PubMed
-
- Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochimica et Biophysica Acta—Biomembranes. 1999;1462: 11–28. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

