Mitochondrial VDAC1-based peptides: Attacking oncogenic properties in glioblastoma
- PMID: 28412744
- PMCID: PMC5458211
- DOI: 10.18632/oncotarget.15455
Mitochondrial VDAC1-based peptides: Attacking oncogenic properties in glioblastoma
Abstract
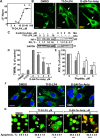
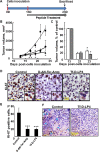
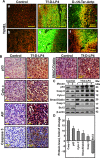
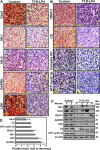
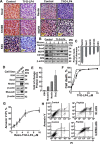
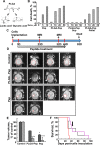
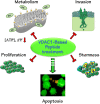
Glioblastoma multiforme (GBM), a primary brain malignancy characterized by high morbidity, invasiveness, proliferation, relapse and mortality, is resistant to chemo- and radiotherapies and lacks effective treatment. GBM tumors undergo metabolic reprograming and develop anti-apoptotic defenses. We targeted GBM with a peptide derived from the mitochondrial protein voltage-dependent anion channel 1 (VDAC1), a key component of cell energy, metabolism and apoptosis regulation. VDAC1-based cell-penetrating peptides perturbed cell energy and metabolic homeostasis and induced apoptosis in several GBM and GBM-derived stem cell lines. We found that the peptides simultaneously attacked several oncogenic properties of human U-87MG cells introduced into sub-cutaneous xenograft mouse model, inhibiting tumor growth, invasion, and cellular metabolism, stemness and inducing apoptosis. Peptide-treated tumors showed decreased expression of all tested metabolism-related enzymes and transporters, and elevated levels of apoptotic proteins, such as p53, cytochrome c and caspases. Retro-Tf-D-LP4, containing the human transferrin receptor (TfR)-recognition sequence, crossed the blood-brain barrier (BBB) via the TfR that is highly expressed in the BBB to strongly inhibit tumor growth in an intracranial xenograft mouse model. In summary, the VDAC1-based peptides tested here offer a potentially affordable and innovative new conceptual therapeutic paradigm that might overcome GBM stemness and invasiveness and reduce relapse rates.
Keywords: VDAC1; apoptosis; glioblastoma; mitochondria; peptides.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
VDAC1-based peptides: novel pro-apoptotic agents and potential therapeutics for B-cell chronic lymphocytic leukemia.Cell Death Dis. 2013 Sep 19;4(9):e809. doi: 10.1038/cddis.2013.316. Cell Death Dis. 2013. PMID: 24052077 Free PMC article.
-
Reducing VDAC1 expression induces a non-apoptotic role for pro-apoptotic proteins in cancer cell differentiation.Biochim Biophys Acta. 2016 Aug;1857(8):1228-1242. doi: 10.1016/j.bbabio.2016.04.005. Epub 2016 Apr 12. Biochim Biophys Acta. 2016. PMID: 27080741
-
Selective induction of cancer cell death by VDAC1-based peptides and their potential use in cancer therapy.Mol Oncol. 2018 Jun;12(7):1077-1103. doi: 10.1002/1878-0261.12313. Epub 2018 May 19. Mol Oncol. 2018. PMID: 29698587 Free PMC article.
-
The mitochondrial voltage-dependent anion channel 1 in tumor cells.Biochim Biophys Acta. 2015 Oct;1848(10 Pt B):2547-75. doi: 10.1016/j.bbamem.2014.10.040. Epub 2014 Nov 4. Biochim Biophys Acta. 2015. PMID: 25448878 Review.
-
VDAC1-Based Peptides as Potential Modulators of VDAC1 Interactions with Its Partners and as a Therapeutic for Cancer, NASH, and Diabetes.Biomolecules. 2024 Sep 9;14(9):1139. doi: 10.3390/biom14091139. Biomolecules. 2024. PMID: 39334905 Free PMC article. Review.
Cited by
-
The VDAC1-based R-Tf-D-LP4 Peptide as a Potential Treatment for Diabetes Mellitus.Cells. 2020 Feb 19;9(2):481. doi: 10.3390/cells9020481. Cells. 2020. PMID: 32093016 Free PMC article.
-
Tumor targeting peptides: novel therapeutic strategies in glioblastoma.Curr Opin Pharmacol. 2019 Aug;47:14-19. doi: 10.1016/j.coph.2019.01.006. Epub 2019 Feb 15. Curr Opin Pharmacol. 2019. PMID: 30776641 Free PMC article. Review.
-
The Multicellular Effects of VDAC1 N-Terminal-Derived Peptide.Biomolecules. 2022 Sep 28;12(10):1387. doi: 10.3390/biom12101387. Biomolecules. 2022. PMID: 36291596 Free PMC article.
-
Peptides as Pharmacological Carriers to the Brain: Promises, Shortcomings and Challenges.Mol Pharm. 2022 Nov 7;19(11):3700-3729. doi: 10.1021/acs.molpharmaceut.2c00523. Epub 2022 Sep 29. Mol Pharm. 2022. PMID: 36174227 Free PMC article. Review.
-
Mitochondrial VDAC1 Silencing in Urethane-Induced Lung Cancer Inhibits Tumor Growth and Alters Cancer Oncogenic Properties.Cancers (Basel). 2024 Aug 26;16(17):2970. doi: 10.3390/cancers16172970. Cancers (Basel). 2024. PMID: 39272828 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

