Antigen Discovery and Therapeutic Targeting in Hematologic Malignancies
- PMID: 28410299
- PMCID: PMC5856170
- DOI: 10.1097/PPO.0000000000000257
Antigen Discovery and Therapeutic Targeting in Hematologic Malignancies
Abstract
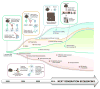
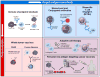
Historically, immune-based therapies have played a leading role in the treatment of hematologic malignancies, with the efficacy of stem cell transplantation largely attributable to donor immunity against malignant cells. As new and more targeted immunotherapies have developed, their role in the treatment of hematologic malignancies is evolving and expanding. Herein, we discuss approaches for antigen discovery and review known and novel tumor antigens in hematologic malignancies. We further explore the role of established and investigational immunotherapies in hematologic malignancies, with a focus on personalization of treatment modalities such as cancer vaccines and adoptive cell therapy. Finally, we identify areas of active investigation and development. Immunotherapy is at an exciting crossroads for the treatment of hematologic malignancies, with further investigation aimed at producing effective, targeted immune therapies that maximize antitumor effects while minimizing toxicity.
Conflict of interest statement
Figures


Similar articles
-
Neoantigens in Hematologic Malignancies.Front Immunol. 2020 Feb 14;11:121. doi: 10.3389/fimmu.2020.00121. eCollection 2020. Front Immunol. 2020. PMID: 32117272 Free PMC article. Review.
-
Hematopoietic system-specific antigens as targets for cellular immunotherapy of hematological malignancies.Semin Hematol. 2002 Jan;39(1):23-31. doi: 10.1053/shem.2002.29248. Semin Hematol. 2002. PMID: 11799526 Review.
-
Evaluation of current cancer immunotherapy: hemato-oncology.Cancer J. 2011 Sep-Oct;17(5):309-24. doi: 10.1097/PPO.0b013e3182341fde. Cancer J. 2011. PMID: 21952281 Free PMC article. Review.
-
Immunotherapy in Hematologic Malignancies: Emerging Therapies and Novel Approaches.Int J Mol Sci. 2020 Oct 27;21(21):8000. doi: 10.3390/ijms21218000. Int J Mol Sci. 2020. PMID: 33121189 Free PMC article. Review.
-
[Cellular immunotherapy for hematological malignancies].Rinsho Ketsueki. 2008 Oct;49(10):1460-71. Rinsho Ketsueki. 2008. PMID: 18833930 Review. Japanese. No abstract available.
Cited by
-
The Transcriptomic Analysis of NSC-34 Motor Neuron-Like Cells Reveals That Cannabigerol Influences Synaptic Pathways: A Comparative Study with Cannabidiol.Life (Basel). 2020 Oct 1;10(10):227. doi: 10.3390/life10100227. Life (Basel). 2020. PMID: 33019509 Free PMC article.
-
In Vitro-Transcribed mRNA Chimeric Antigen Receptor T Cell (IVT mRNA CAR T) Therapy in Hematologic and Solid Tumor Management: A Preclinical Update.Int J Mol Sci. 2020 Sep 6;21(18):6514. doi: 10.3390/ijms21186514. Int J Mol Sci. 2020. PMID: 32899932 Free PMC article. Review.
-
Engineered tissues and strategies to overcome challenges in drug development.Adv Drug Deliv Rev. 2020;158:116-139. doi: 10.1016/j.addr.2020.09.012. Epub 2020 Sep 26. Adv Drug Deliv Rev. 2020. PMID: 32987094 Free PMC article.
-
Beyond conventional immune-checkpoint inhibition - novel immunotherapies for renal cell carcinoma.Nat Rev Clin Oncol. 2021 Apr;18(4):199-214. doi: 10.1038/s41571-020-00455-z. Epub 2021 Jan 12. Nat Rev Clin Oncol. 2021. PMID: 33437048 Free PMC article. Review.
-
Adoptive Immunotherapy with Antigen-Specific T Cells Expressing a Native TCR.Cancer Immunol Res. 2019 Apr;7(4):528-533. doi: 10.1158/2326-6066.CIR-18-0888. Cancer Immunol Res. 2019. PMID: 30936089 Free PMC article. Review.
References
-
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. - PubMed
-
- Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–5. - PubMed
-
- Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

