The suppression of apoptosis by α-herpesvirus
- PMID: 28406478
- PMCID: PMC5477576
- DOI: 10.1038/cddis.2017.139
The suppression of apoptosis by α-herpesvirus
Abstract
Apoptosis, an important innate immune mechanism that eliminates pathogen-infected cells, is primarily triggered by two signalling pathways: the death receptor pathway and the mitochondria-mediated pathway. However, many viruses have evolved various strategies to suppress apoptosis by encoding anti-apoptotic factors or regulating apoptotic signalling pathways, which promote viral propagation and evasion of the host defence. During its life cycle, α-herpesvirus utilizes an elegant multifarious anti-apoptotic strategy to suppress programmed cell death. This progress article primarily focuses on the current understanding of the apoptosis-inhibition mechanisms of α-herpesvirus anti-apoptotic genes and their expression products and discusses future directions, including how the anti-apoptotic function of herpesvirus could be targeted therapeutically.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
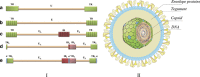
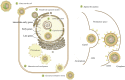
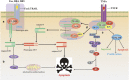
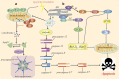
Similar articles
-
Human herpesvirus 8 interferon regulatory factor-mediated BH3-only protein inhibition via Bid BH3-B mimicry.PLoS Pathog. 2012;8(6):e1002748. doi: 10.1371/journal.ppat.1002748. Epub 2012 Jun 7. PLoS Pathog. 2012. PMID: 22685405 Free PMC article.
-
Investigating the biology of alpha herpesviruses with MS-based proteomics.Proteomics. 2015 Jun;15(12):1943-56. doi: 10.1002/pmic.201400604. Epub 2015 May 15. Proteomics. 2015. PMID: 25764121 Free PMC article. Review.
-
Bim nuclear translocation and inactivation by viral interferon regulatory factor.PLoS Pathog. 2010 Aug 5;6(8):e1001031. doi: 10.1371/journal.ppat.1001031. PLoS Pathog. 2010. PMID: 20700448 Free PMC article.
-
Evasion of the Cell-Mediated Immune Response by Alphaherpesviruses.Viruses. 2020 Nov 26;12(12):1354. doi: 10.3390/v12121354. Viruses. 2020. PMID: 33256093 Free PMC article. Review.
-
The interplay between human herpes simplex virus infection and the apoptosis and necroptosis cell death pathways.Virol J. 2016 May 6;13:77. doi: 10.1186/s12985-016-0528-0. Virol J. 2016. PMID: 27154074 Free PMC article. Review.
Cited by
-
Duck plague virus US3 protein kinase phosphorylates UL47 and regulates the subcellular localization of UL47.Front Microbiol. 2022 Oct 25;13:876820. doi: 10.3389/fmicb.2022.876820. eCollection 2022. Front Microbiol. 2022. PMID: 36386680 Free PMC article.
-
Granzyme B Cleaves Multiple Herpes Simplex Virus 1 and Varicella-Zoster Virus (VZV) Gene Products, and VZV ORF4 Inhibits Natural Killer Cell Cytotoxicity.J Virol. 2019 Oct 29;93(22):e01140-19. doi: 10.1128/JVI.01140-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462576 Free PMC article.
-
Mammalian orthoreovirus capsid protein σ3 antagonizes RLR-mediated antiviral responses by degrading MAVS.mSphere. 2024 Jun 25;9(6):e0023624. doi: 10.1128/msphere.00236-24. Epub 2024 May 17. mSphere. 2024. PMID: 38757961 Free PMC article.
-
Apoptosis in the late replication phase of Bovine alphaherpesvirus 1 in experimentally infected calves.Braz J Microbiol. 2021 Dec;52(4):2529-2534. doi: 10.1007/s42770-021-00546-8. Epub 2021 Aug 6. Braz J Microbiol. 2021. PMID: 34355356 Free PMC article.
-
Mitochondrial Oxidative Phosphorylation in Viral Infections.Viruses. 2023 Dec 4;15(12):2380. doi: 10.3390/v15122380. Viruses. 2023. PMID: 38140621 Free PMC article. Review.
References
-
- Pellett P, Roizman B. Herpesviridae. In: Knipe DM, Howley PM (eds). Fields Virology. 6th edn. Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2013, pp 1802–1822.
-
- Gray WL, Starnes B, White MW, Mahalingam R. The DNA sequence of the simian varicella virus genome. Virology 2001; 284: 123–130. - PubMed
-
- Waltzek TB, Kelley GO, Stone DM, Way K, Hanson L, Fukuda H et al. Koi herpesvirus represents a third cyprinid herpesvirus (CyHV-3) in the family Herpesviridae. J Gen Virol 2005; 86: 1659–1667. - PubMed
-
- Davison AJ. Herpesviruses: general features. In Encyclopedia of Virology. 3rd edn. Brian WJ Mahy, Marc HV Van (eds). Regenmortel, Academic Press: Oxford, 2008, p 430–436.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

