Inhibition of neuronal ferroptosis protects hemorrhagic brain
- PMID: 28405617
- PMCID: PMC5374066
- DOI: 10.1172/jci.insight.90777
Inhibition of neuronal ferroptosis protects hemorrhagic brain
Abstract
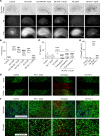
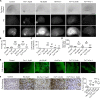
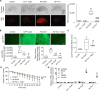
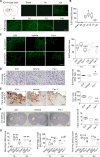
Intracerebral hemorrhage (ICH) causes high mortality and morbidity, but our knowledge of post-ICH neuronal death and related mechanisms is limited. In this study, we first demonstrated that ferroptosis, a newly identified form of cell death, occurs in the collagenase-induced ICH model in mice. We found that administration of ferrostatin-1, a specific inhibitor of ferroptosis, prevented neuronal death and reduced iron deposition induced by hemoglobin in organotypic hippocampal slice cultures (OHSCs). Mice treated with ferrostatin-1 after ICH exhibited marked brain protection and improved neurologic function. Additionally, we found that ferrostatin-1 reduced lipid reactive oxygen species production and attenuated the increased expression level of PTGS2 and its gene product cyclooxygenase-2 ex vivo and in vivo. Moreover, ferrostatin-1 in combination with other inhibitors that target different forms of cell death prevented hemoglobin-induced cell death in OHSCs and human induced pluripotent stem cell-derived neurons better than any inhibitor alone. These results indicate that ferroptosis contributes to neuronal death after ICH, that administration of ferrostatin-1 protects hemorrhagic brain, and that cyclooxygenase-2 could be a biomarker of ferroptosis. The insights gained from this study will advance our knowledge of the post-ICH cell death cascade and be essential for future preclinical studies.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures

Similar articles
-
Inhibition of neuronal ferroptosis in the acute phase of intracerebral hemorrhage shows long-term cerebroprotective effects.Brain Res Bull. 2019 Nov;153:122-132. doi: 10.1016/j.brainresbull.2019.08.013. Epub 2019 Aug 20. Brain Res Bull. 2019. PMID: 31442590
-
The 5-Lipoxygenase Inhibitor Zileuton Confers Neuroprotection against Glutamate Oxidative Damage by Inhibiting Ferroptosis.Biol Pharm Bull. 2015;38(8):1234-9. doi: 10.1248/bpb.b15-00048. Biol Pharm Bull. 2015. PMID: 26235588
-
Ferrostatin-1 Polarizes Microglial Cells Toward M2 Phenotype to Alleviate Inflammation After Intracerebral Hemorrhage.Neurocrit Care. 2022 Jun;36(3):942-954. doi: 10.1007/s12028-021-01401-2. Epub 2022 Jan 31. Neurocrit Care. 2022. PMID: 35099711
-
Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage.Stroke Vasc Neurol. 2019 Jan 13;4(2):93-95. doi: 10.1136/svn-2018-000205. eCollection 2019 Jul. Stroke Vasc Neurol. 2019. PMID: 31338218 Free PMC article. Review.
-
Ferroptosis, a Regulated Neuronal Cell Death Type After Intracerebral Hemorrhage.Front Cell Neurosci. 2020 Nov 16;14:591874. doi: 10.3389/fncel.2020.591874. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33304242 Free PMC article. Review.
Cited by
-
Pivotal Role of GSTO2 in Ferroptotic Neuronal Injury After Intracerebral Hemorrhage.J Mol Neurosci. 2024 Feb 22;74(1):24. doi: 10.1007/s12031-023-02187-y. J Mol Neurosci. 2024. PMID: 38386166 Free PMC article.
-
YAP1 protects against septic liver injury via ferroptosis resistance.Cell Biosci. 2022 Oct 1;12(1):163. doi: 10.1186/s13578-022-00902-7. Cell Biosci. 2022. PMID: 36182901 Free PMC article.
-
Iron Dyshomeostasis and Ferroptosis: A New Alzheimer's Disease Hypothesis?Front Aging Neurosci. 2022 Mar 22;14:830569. doi: 10.3389/fnagi.2022.830569. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35391749 Free PMC article. Review.
-
Epigenetic Regulation of Ferroptosis in Central Nervous System Diseases.Mol Neurobiol. 2023 Jul;60(7):3584-3599. doi: 10.1007/s12035-023-03267-1. Epub 2023 Feb 27. Mol Neurobiol. 2023. PMID: 36847936 Review.
-
Wild Bitter Melon Extract Abrogates Hypoxia-Induced Cell Death via the Regulation of Ferroptosis, ER Stress, and Apoptosis in Microglial BV2 Cells.Evid Based Complement Alternat Med. 2022 Mar 16;2022:1072600. doi: 10.1155/2022/1072600. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Dec 6;2023:9851268. doi: 10.1155/2023/9851268 PMID: 35449822 Free PMC article. Retracted.
References
-
- Wasserman JK, Schlichter LC. Neuron death and inflammation in a rat model of intracerebral hemorrhage: effects of delayed minocycline treatment. Brain Res. 2007;1136(1):208–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

