miR-29a regulates the proliferation and differentiation of retinal progenitors by targeting Rbm8a
- PMID: 28404883
- PMCID: PMC5458264
- DOI: 10.18632/oncotarget.16669
miR-29a regulates the proliferation and differentiation of retinal progenitors by targeting Rbm8a
Abstract
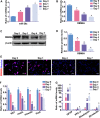
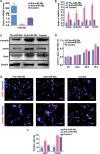
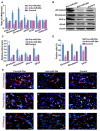
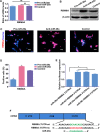
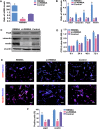
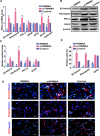
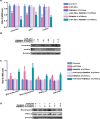
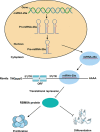
During development, tight regulation of the expansion of retinal progenitor cells (RPCs) and their differentiation into neuronal and glial cells is important for retinal formation and function. Our study demonstrated that microRNA (miR)-29a modulated the proliferation and differentiation of RPCs by suppressing RBM8A (one of the factors in the exon junction complex). Particularly, overexpression of miR-29a reduced RPC proliferation but accelerated RPC differentiation. By contrast, reduction of endogenous miR-29a elicited the opposite effects. Overexpression of miR-29a repressed the translation of Rbm8a, thus negatively regulating RPC proliferation and promoting the neuronal and glial differentiation of RPCs, and knockdown of endogenous Rbm8a phenocopied the observed effects of miR-29a overexpression. Furthermore, a luciferase reporter assay showed that miR-29a directly interacted with the Rbm8a mRNA 3'UTR, which indicated that Rbm8a is the direct target of miR-29a. To further verify the result, co-overexpression of the Rbm8a 3' UTR-wt (plasmids into which the Rbm8a 3' UTR sequence had been introduced) and miR-29a in RPCs rescued the phenotype associated with miR-29a overexpression, reversing the promotion of differentiation and inhibition of proliferation. These results show a novel mechanism by which miR-29a regulates the proliferation and differentiation of RPCs through Rbm8a.
Keywords: RBM8A; differentiation; microRNA (miR)-29a; proliferation; retinal progenitor cells.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
Similar articles
-
miR-762 regulates the proliferation and differentiation of retinal progenitor cells by targeting NPDC1.Cell Cycle. 2020 Jul;19(14):1754-1767. doi: 10.1080/15384101.2020.1777805. Epub 2020 Jun 16. Cell Cycle. 2020. PMID: 32544377 Free PMC article.
-
Reciprocal actions of microRNA-9 and TLX in the proliferation and differentiation of retinal progenitor cells.Stem Cells Dev. 2014 Nov 15;23(22):2771-81. doi: 10.1089/scd.2014.0021. Epub 2014 Jul 14. Stem Cells Dev. 2014. PMID: 24901604
-
REST, regulated by RA through miR-29a and the proteasome pathway, plays a crucial role in RPC proliferation and differentiation.Cell Death Dis. 2018 May 1;9(5):444. doi: 10.1038/s41419-018-0473-5. Cell Death Dis. 2018. PMID: 29670089 Free PMC article.
-
Overexpression of miRNA29a gene inhibits proliferation and promotes apoptosis of jejunal epithelial cells in yak.Anim Biotechnol. 2024 Nov;35(1):2391520. doi: 10.1080/10495398.2024.2391520. Epub 2024 Sep 2. Anim Biotechnol. 2024. PMID: 39222080 Review.
-
Pluripotent stem cell-derived somatic stem cells as tool to study the role of microRNAs in early human neural development.Curr Mol Med. 2013 Jun;13(5):707-22. doi: 10.2174/1566524011313050003. Curr Mol Med. 2013. PMID: 23642053 Review.
Cited by
-
miR-381-3p Cooperated With Hes1 to Regulate the Proliferation and Differentiation of Retinal Progenitor Cells.Front Cell Dev Biol. 2022 Feb 25;10:853215. doi: 10.3389/fcell.2022.853215. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35281083 Free PMC article.
-
Prognostic value of increased expression of RBM8A in gastric cancer.Braz J Med Biol Res. 2020 Apr 9;53(4):e9290. doi: 10.1590/1414-431X20209290. eCollection 2020. Braz J Med Biol Res. 2020. PMID: 32294703 Free PMC article.
-
Therapeutic effects of mesenchymal stem cells-derived extracellular vesicles' miRNAs on retinal regeneration: a review.Stem Cell Res Ther. 2021 Oct 7;12(1):530. doi: 10.1186/s13287-021-02588-z. Stem Cell Res Ther. 2021. PMID: 34620234 Free PMC article. Review.
-
miR‑29a‑3p regulates the epithelial‑mesenchymal transition via the SPARC/ERK signaling pathway in human bronchial epithelial cells.Int J Mol Med. 2021 Sep;48(3):171. doi: 10.3892/ijmm.2021.5004. Epub 2021 Jul 19. Int J Mol Med. 2021. PMID: 34278471 Free PMC article.
-
Interplay of RNA-Binding Proteins and microRNAs in Neurodegenerative Diseases.Int J Mol Sci. 2021 May 18;22(10):5292. doi: 10.3390/ijms22105292. Int J Mol Sci. 2021. PMID: 34069857 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

