Enhancement of trophoblast differentiation and survival by low molecular weight heparin requires heparin-binding EGF-like growth factor
- PMID: 28402449
- PMCID: PMC6075585
- DOI: 10.1093/humrep/dex069
Enhancement of trophoblast differentiation and survival by low molecular weight heparin requires heparin-binding EGF-like growth factor
Abstract
Study question: Does low molecular weight heparin (LMWH) require heparin-binding epidermal growth factor (EGF)-like growth factor (HBEGF) signaling to induce extravillous trophoblast differentiation and decrease apoptosis during oxidative stress?
Summary answer: LMWH increased HBEGF expression and secretion, and HBEGF signaling was required to stimulate trophoblast extravillous differentiation, increase invasion in vitro and reduce trophoblast apoptosis during oxidative stress.
What is known already: Abnormal trophoblast differentiation and survival contribute to placental insufficiency syndromes, including preeclampsia and intrauterine growth restriction. Preeclampsia often manifests as a pro-thrombotic state, with unsuccessful transformation of the spiral arteries that reduces oxygen supply and can produce placental infarction. LMWH improves placental function by increasing blood flow. Recent data suggest that the actions of LMWH transcend its anti-coagulative properties, but the molecular mechanism is unknown. There is evidence that LMWH alters the expression of human HBEGF in trophoblast cells, which regulates human trophoblast pathophysiology. HBEGF, itself, is capable of increasing trophoblast survival and invasiveness.
Study design, size, duration: First-trimester placental explants and the HTR-8/SVneo cell line, established using extravillous trophoblast outgrowths from first-trimester villous explants, were treated in vitro with LMWH to examine the effects on HBEGF signaling and trophoblast function under normal physiological and pathological conditions. A highly specific antagonist of HBEGF and other inhibitors of HBEGF downstream signaling were used to determine the relationship between LMWH treatment and HBEGF.
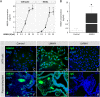
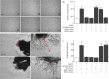
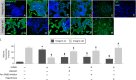
Participants/materials, setting, methods: Placental tissues (n = 5) were obtained with IRB approval and patient consent from first-trimester terminations. Placental explants and HTR-8/SVneo cells were cultured on plastic or Matrigel™ and treated with a therapeutic dose of LMWH (Enoxaparin; 10 IU/ml), with or without CRM197, pan Erb-B2 Receptor Tyrosine Kinase (ERBB) inhibitor, anti-ERBB1 or ERBB4 blocking antibodies, or pretreatment of cells with heparitinase I. Extravillous differentiation was assessed by immunocytochemistry to determine the relative levels of integrins α6β4 and α1β1. Trophoblast invasiveness was assessed in villous explants by measuring outgrowth from villous tips cultured on Matrigel, and by invasion assays with HTR-8/SVneo cells cultured on Matrigel-coated transwell insert. Placental explants and HTR-8/SVneo cells were exposed to oxidative stress in a hypoxia-reoxygenation (H-R) model, measuring cell death by TUNEL assay, caspase 3 cleavage, and BCL-2α expression.
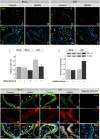
Main results and the role of chance: LMWH induced extravillous differentiation, according to trophoblast invasion assays and integrin (α6β4-α1β1) switching. Treatment with LMWH rescued cytotrophoblasts and HTR-8/SVneo cells from apoptosis during exposure to reoxygenation injury, based on TUNEL, caspase 3 cleavage and BCL-2α expression. Experiments using CRM197, ERBB1 and ERBB4 blocking antibodies, pan-ERBB inhibitor and removal of cell surface heparin demonstrated that the effects of LMWH on trophoblast invasion and survival were dependent upon HBEGF signaling.
Large scale data: N/A.
Limitations, reasons for caution: The primary limitation of this study was the use of only in vitro experiments. Patient demographics from elective terminations were not available.
Wider implications of the findings: These data provide new insights into the non-coagulation-related aspects of perinatal LMWH treatment in the management of placental insufficiency disorders.
Study funding/competing interest(s): This research was supported by grants from the National Institutes of Health (HD071408 and HL128628), the March of Dimes, and the W. K. Kellogg Foundation. There were no conflicts or competing interests.
Keywords: BCL-2α; HBEGFimplantation; LMWH; apoptosis; caspase; differentiation; integrins; placenta; trophoblast.
© The Author 2017. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com
Figures
Similar articles
-
Trophoblast subtype-specific EGFR/ERBB4 expression correlates with cell cycle progression and hyperplasia in complete hydatidiform moles.Hum Reprod. 2015 Apr;30(4):789-99. doi: 10.1093/humrep/dev027. Epub 2015 Mar 3. Hum Reprod. 2015. PMID: 25740878
-
ADAM8 localizes to extravillous trophoblasts within the maternal-fetal interface and potentiates trophoblast cell line migration through a β1 integrin-mediated mechanism.Mol Hum Reprod. 2018 Oct 1;24(10):495-509. doi: 10.1093/molehr/gay034. Mol Hum Reprod. 2018. PMID: 30124911 Free PMC article.
-
Emerging nonanticoagulant role of low molecular weight heparins on extravillous trophoblast functions and on heparin binding-epidermal growth factor and cystein-rich angiogenic inducer 61 expression.Fertil Steril. 2012 Oct;98(4):1028-36.e1-2. doi: 10.1016/j.fertnstert.2012.06.042. Epub 2012 Jul 19. Fertil Steril. 2012. PMID: 22818289
-
TGFβ signalling: a nexus between inflammation, placental health and preeclampsia throughout pregnancy.Hum Reprod Update. 2024 Jul 1;30(4):442-471. doi: 10.1093/humupd/dmae007. Hum Reprod Update. 2024. PMID: 38519450 Free PMC article. Review.
-
Investigation of human trophoblast invasion in vitro.Hum Reprod Update. 2020 Jun 18;26(4):501-513. doi: 10.1093/humupd/dmaa017. Hum Reprod Update. 2020. PMID: 32441309 Free PMC article. Review.
Cited by
-
Cooperation between NSPc1 and DNA methylation represses HOXA11 expression and promotes apoptosis of trophoblast cells during preeclampsia.Acta Biochim Biophys Sin (Shanghai). 2023 Feb 25;55(2):1-13. doi: 10.3724/abbs.2023012. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 36815373 Free PMC article.
-
LMWH prevents thromboinflammation in the placenta via HBEGF-AKT signaling.Blood Adv. 2024 Sep 24;8(18):4756-4766. doi: 10.1182/bloodadvances.2023011895. Blood Adv. 2024. PMID: 38941535 Free PMC article.
-
Effects of glycol-split low molecular weight heparin on placental, endothelial, and anti-inflammatory pathways relevant to preeclampsia.Biol Reprod. 2018 Nov 1;99(5):1082-1090. doi: 10.1093/biolre/ioy127. Biol Reprod. 2018. PMID: 29860275 Free PMC article.
-
Efficacy of Low Molecular Heparin on Preeclampsia by Inhibiting Apoptosis of Trophoblasts via the p38MAPK Signaling Pathway.Comput Math Methods Med. 2021 Aug 2;2021:3337514. doi: 10.1155/2021/3337514. eCollection 2021. Comput Math Methods Med. 2021. Retraction in: Comput Math Methods Med. 2023 Aug 30;2023:9794675. doi: 10.1155/2023/9794675. PMID: 34394705 Free PMC article. Retracted.
-
The Ontogeny and Function of Placental Macrophages.Front Immunol. 2021 Oct 21;12:771054. doi: 10.3389/fimmu.2021.771054. eCollection 2021. Front Immunol. 2021. PMID: 34745147 Free PMC article. Review.
References
-
- Adiguzel C, Jeske WP, Hoppensteadt D, Walenga JM, Bansal V, Fareed J. Structural and functional characterization of low-molecular-weight heparins: impact on the development of guidelines for generic products. Clin Appl Thromb Hemost 2009;15:137–144. - PubMed
-
- Aghajanova L, Shen S, Rojas AM, Fisher SJ, Irwin JC, Giudice LC. Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol Reprod 2012;86:1–21. - PubMed
-
- Akhtar MA, Sur S, Raine-Fenning N, Jayaprakasan K, Thornton J, Quenby S, Marjoribanks J. Heparin for assisted reproduction: summary of a Cochrane review. Fertil Steril 2015;103:33–34. - PubMed
-
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol 2000;96:271–276. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

