From Molecular Mechanisms to Clinical Management of Antineoplastic Drug-Induced Cardiovascular Toxicity: A Translational Overview
- PMID: 28398124
- PMCID: PMC6529857
- DOI: 10.1089/ars.2016.6930
From Molecular Mechanisms to Clinical Management of Antineoplastic Drug-Induced Cardiovascular Toxicity: A Translational Overview
Abstract
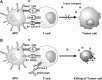
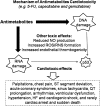
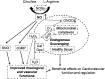
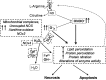
Significance: Antineoplastic therapies have significantly improved the prognosis of oncology patients. However, these treatments can bring to a higher incidence of side-effects, including the worrying cardiovascular toxicity (CTX). Recent Advances: Substantial evidence indicates multiple mechanisms of CTX, with redox mechanisms playing a key role. Recent data singled out mitochondria as key targets for antineoplastic drug-induced CTX; understanding the underlying mechanisms is, therefore, crucial for effective cardioprotection, without compromising the efficacy of anti-cancer treatments. Critical Issues: CTX can occur within a few days or many years after treatment. Type I CTX is associated with irreversible cardiac cell injury, and it is typically caused by anthracyclines and traditional chemotherapeutics. Type II CTX is generally caused by novel biologics and more targeted drugs, and it is associated with reversible myocardial dysfunction. Therefore, patients undergoing anti-cancer treatments should be closely monitored, and patients at risk of CTX should be identified before beginning treatment to reduce CTX-related morbidity. Future Directions: Genetic profiling of clinical risk factors and an integrated approach using molecular, imaging, and clinical data may allow the recognition of patients who are at a high risk of developing chemotherapy-related CTX, and it may suggest methodologies to limit damage in a wider range of patients. The involvement of redox mechanisms in cancer biology and anticancer treatments is a very active field of research. Further investigations will be necessary to uncover the hallmarks of cancer from a redox perspective and to develop more efficacious antineoplastic therapies that also spare the cardiovascular system.
Keywords: ErbB2 inhibitors; cancer immunotherapy; chemotherapy; oxidative/nitrosative stress; tyrosine kinase inhibitors; vascular endothelial growth factor.
Figures

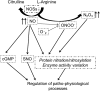
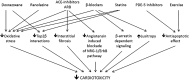

Similar articles
-
Antineoplastic Drug-Induced Cardiotoxicity: A Redox Perspective.Front Physiol. 2018 Mar 7;9:167. doi: 10.3389/fphys.2018.00167. eCollection 2018. Front Physiol. 2018. PMID: 29563880 Free PMC article. Review.
-
Oxidative stress in anticancer therapies-related cardiac dysfunction.Free Radic Biol Med. 2021 Jun;169:410-415. doi: 10.1016/j.freeradbiomed.2021.04.021. Epub 2021 Apr 28. Free Radic Biol Med. 2021. PMID: 33930514
-
Emerging mitochondrial signaling mechanisms in cardio-oncology: beyond oxidative stress.Am J Physiol Heart Circ Physiol. 2022 Oct 1;323(4):H702-H720. doi: 10.1152/ajpheart.00231.2022. Epub 2022 Aug 5. Am J Physiol Heart Circ Physiol. 2022. PMID: 35930448 Free PMC article. Review.
-
Role of oxidative stress in cardiotoxicity of antineoplastic drugs.Life Sci. 2019 Sep 1;232:116526. doi: 10.1016/j.lfs.2019.06.001. Epub 2019 Jun 3. Life Sci. 2019. PMID: 31170418 Review.
-
Improving the preclinical models for the study of chemotherapy-induced cardiotoxicity: a Position Paper of the Italian Working Group on Drug Cardiotoxicity and Cardioprotection.Heart Fail Rev. 2015 Sep;20(5):621-31. doi: 10.1007/s10741-015-9497-4. Heart Fail Rev. 2015. PMID: 26168714
Cited by
-
PET Radiopharmaceuticals for Imaging Chemotherapy-Induced Cardiotoxicity.Curr Cardiol Rep. 2020 Jun 19;22(8):62. doi: 10.1007/s11886-020-01315-z. Curr Cardiol Rep. 2020. PMID: 32562004 Free PMC article. Review.
-
Molecular Mechanisms of Antiproliferative Effects of Natural Chalcones.Cancers (Basel). 2021 May 31;13(11):2730. doi: 10.3390/cancers13112730. Cancers (Basel). 2021. PMID: 34073042 Free PMC article. Review.
-
Evaluation of skeletal muscle function in male rats with doxorubicin-induced myopathy following various exercise techniques: the significant role of glucose transporter 4.Pflugers Arch. 2024 May;476(5):797-808. doi: 10.1007/s00424-024-02922-3. Epub 2024 Feb 17. Pflugers Arch. 2024. PMID: 38368293 Free PMC article.
-
MicroRNAs in Cancer Treatment-Induced Cardiotoxicity.Cancers (Basel). 2020 Mar 17;12(3):704. doi: 10.3390/cancers12030704. Cancers (Basel). 2020. PMID: 32192047 Free PMC article. Review.
-
PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy.Front Pharmacol. 2021 Sep 1;12:731798. doi: 10.3389/fphar.2021.731798. eCollection 2021. Front Pharmacol. 2021. PMID: 34539412 Free PMC article. Review.
References
-
- Abd El-Aziz MA, Othman AI, Amer M, and El-Missiry MA. Potential protective role of angiotensin-converting enzyme inhibitors captopril and enalapril against adriamycin-induced acute cardiac and hepatic toxicity in rats. J Appl Toxicol 21: 469–473, 2001 - PubMed
-
- Aichberger KJ, Herndlhofer S, Schernthaner GH, Schillinger M, Mitterbauer-Hohendanner G, Sillaber C, and Valent P. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol 86: 533–539, 2011 - PubMed
-
- Alhawiti N, Burbury KL, Kwa FA, O'Malley CJ, Shuttleworth P, Alzard M, Hamadi A, Grigg AP, and Jackson DE. The tyrosine kinase inhibitor, nilotinib potentiates a prothrombotic state. Thromb Res 145: 54–64, 2016 - PubMed
-
- Alter P, Herzum M, Soufi M, Schaefer JR, and Maisch B. Cardiotoxicity of 5-fluorouracil. Cardiovasc Hematol Agents Med Chem 4: 1–5, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

