RNA Interference-Induced Innate Immunity, Off-Target Effect, or Immune Adjuvant?
- PMID: 28386261
- PMCID: PMC5362589
- DOI: 10.3389/fimmu.2017.00331
RNA Interference-Induced Innate Immunity, Off-Target Effect, or Immune Adjuvant?
Abstract
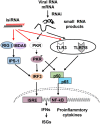
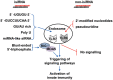
RNA interference (RNAi) is a natural cellular mechanism that inhibits gene expression in a sequence-specific manner. In the last decade, RNAi has become a cornerstone in basic biological systems research and drug development efforts. The RNAi-based manipulation of mammalian cells facilitates target identification and validation; assists in identifying human disease etiologies; and expedites the development of treatments for infectious diseases, cancer, and other conditions. Several RNAi-based approaches are currently undergoing assessment in phase I and II clinical trials. However, RNAi-associated immune stimulation might act as a hurdle to safe and effective RNAi, particularly in clinical applications. The induction of innate immunity may originate from small interfering RNA (siRNA) sequence-dependent delivery vehicles and even the RNAi process itself. However, in the case of antagonistic cancers and viral infection, immune activation is beneficial; thus, immunostimulatory small interfering RNAs were designed to create bifunctional small molecules with RNAi and immunostimulatory activities. This review summarizes the research studies of RNAi-associated immune stimulation and the approaches for manipulating immunostimulatory activities.
Keywords: RNA interference; immunostimulatory RNA; immunostimulatory small interfering RNAs; innate immunity; small interfering RNA.
Figures
Similar articles
-
Antiviral innate immune response of RNA interference.J Infect Dev Ctries. 2014 Jul 14;8(7):804-10. doi: 10.3855/jidc.4187. J Infect Dev Ctries. 2014. PMID: 25022288 Review.
-
Mechanisms of immune system activation in mammalians by small interfering RNA (siRNA).Artif Cells Nanomed Biotechnol. 2016 Nov;44(7):1589-96. doi: 10.3109/21691401.2015.1102738. Epub 2015 Oct 26. Artif Cells Nanomed Biotechnol. 2016. PMID: 26497011 Review.
-
Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA.Nat Biotechnol. 2005 Apr;23(4):457-62. doi: 10.1038/nbt1081. Epub 2005 Mar 20. Nat Biotechnol. 2005. PMID: 15778705
-
shRNA-induced interferon-stimulated gene analysis.Methods Mol Biol. 2012;820:163-77. doi: 10.1007/978-1-61779-439-1_10. Methods Mol Biol. 2012. PMID: 22131031
-
Silencing or stimulation? siRNA delivery and the immune system.Annu Rev Chem Biomol Eng. 2011;2:77-96. doi: 10.1146/annurev-chembioeng-061010-114133. Annu Rev Chem Biomol Eng. 2011. PMID: 22432611 Review.
Cited by
-
miRNA therapeutics in precision oncology: a natural premium to nurture.Explor Target Antitumor Ther. 2022;3(4):511-532. doi: 10.37349/etat.2022.00098. Epub 2022 Aug 31. Explor Target Antitumor Ther. 2022. PMID: 36071981 Free PMC article. Review.
-
The various role of microRNAs in breast cancer angiogenesis, with a special focus on novel miRNA-based delivery strategies.Cancer Cell Int. 2023 Feb 10;23(1):24. doi: 10.1186/s12935-022-02837-y. Cancer Cell Int. 2023. PMID: 36765409 Free PMC article. Review.
-
Tra2beta-Dependent Regulation of RIO Kinase 3 Splicing During Rift Valley Fever Virus Infection Underscores the Links Between Alternative Splicing and Innate Antiviral Immunity.Front Cell Infect Microbiol. 2022 Jan 19;11:799024. doi: 10.3389/fcimb.2021.799024. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35127560 Free PMC article.
-
Intervertebral disc degeneration-Current therapeutic options and challenges.Front Public Health. 2023 Jul 6;11:1156749. doi: 10.3389/fpubh.2023.1156749. eCollection 2023. Front Public Health. 2023. PMID: 37483952 Free PMC article. Review.
-
Therapeutic efficacy of a novel self-assembled immunostimulatory siRNA combining apoptosis promotion with RIG-I activation in gliomas.J Transl Med. 2024 Apr 29;22(1):395. doi: 10.1186/s12967-024-05151-5. J Transl Med. 2024. PMID: 38685028 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

