Visualization of a neurotropic flavivirus infection in mouse reveals unique viscerotropism controlled by host type I interferon signaling
- PMID: 28382163
- PMCID: PMC5381253
- DOI: 10.7150/thno.16615
Visualization of a neurotropic flavivirus infection in mouse reveals unique viscerotropism controlled by host type I interferon signaling
Abstract
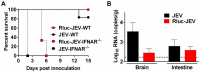
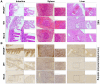
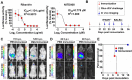
Flavivirus includes a large group of human pathogens with medical importance. Especially, neurotropic flaviviruses capable of invading central and peripheral nervous system, e.g. Japanese encephalitis virus (JEV) and Zika virus (ZIKV), are highly pathogenic to human and constitute major global health problems. However, the dynamic dissemination and pathogenesis of neurotropic flavivirus infections remain largely unknown. Here, using JEV as a model, we rationally designed and constructed a recombinant reporter virus that stably expressed Renilla luciferase (Rluc). The resulting JEV reporter virus (named Rluc-JEV) and parental JEV exhibited similar replication and infection characteristics, and the magnitude of Rluc activity correlated well with progeny viral production in vitro and in vivo. By using in vivo bioluminescence imaging (BLI) technology, we dissected the replication and dissemination dynamics of JEV infection in mice upon different inoculation routes. Interestingly, besides replicating in mouse brain, Rluc-JEV predominantly invaded the abdominal organs in mice with typical viscerotropism. Further tests in mice deficient in type I interferon (IFN) receptors demonstrated robust and prolonged viral replication in the intestine, spleen, liver, kidney and other abdominal organs. Combined with histopathological and immunohistochemical results, the host type I IFN signaling was evidenced as the major barrier to the viscerotropism and pathogenicity of this neurotropic flavivirus. Additionally, the Rluc-JEV platform was readily adapted for efficacy assay of known antiviral compounds and a live JE vaccine. Collectively, our study revealed abdominal organs as important targets of JEV infection in mice and profiled the unique viscerotropism trait controlled by the host type I IFN signaling. This in vivo visualization technology described here provides a powerful tool for testing antiviral agents and vaccine candidates for flaviviral infection.
Keywords: Bioluminescence imaging; Flavivirus; Interferon signaling.; Japanese encephalitis virus; Mouse.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Type I IFN signaling limits hemorrhage-like disease after infection with Japanese encephalitis virus through modulating a prerequisite infection of CD11b+Ly-6C+ monocytes.J Neuroinflammation. 2021 Jun 15;18(1):136. doi: 10.1186/s12974-021-02180-5. J Neuroinflammation. 2021. PMID: 34130738 Free PMC article.
-
Screening of FDA-Approved Drugs for Inhibitors of Japanese Encephalitis Virus Infection.J Virol. 2017 Oct 13;91(21):e01055-17. doi: 10.1128/JVI.01055-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28814523 Free PMC article.
-
A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection.J Virol. 2017 Mar 29;91(8):e02388-16. doi: 10.1128/JVI.02388-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148804 Free PMC article.
-
Flavivirus encephalitis: pathological aspects of mouse and other animal models.Vet Pathol. 2010 Sep;47(5):806-18. doi: 10.1177/0300985810372507. Epub 2010 Jun 15. Vet Pathol. 2010. PMID: 20551474 Review.
-
Astrocytes in Flavivirus Infections.Int J Mol Sci. 2019 Feb 6;20(3):691. doi: 10.3390/ijms20030691. Int J Mol Sci. 2019. PMID: 30736273 Free PMC article. Review.
Cited by
-
Novel Approach for Insertion of Heterologous Sequences into Full-Length ZIKV Genome Results in Superior Level of Gene Expression and Insert Stability.Viruses. 2020 Jan 3;12(1):61. doi: 10.3390/v12010061. Viruses. 2020. PMID: 31947825 Free PMC article.
-
In-vivo monitoring of infectious diseases in living animals using bioluminescence imaging.Virulence. 2018 Jan 1;9(1):28-63. doi: 10.1080/21505594.2017.1371897. Epub 2017 Dec 8. Virulence. 2018. PMID: 28960132 Free PMC article. Review.
-
Visualizing lymphocytic choriomeningitis virus infection in cells and living mice.iScience. 2022 Sep 7;25(10):105090. doi: 10.1016/j.isci.2022.105090. eCollection 2022 Oct 21. iScience. 2022. PMID: 36185356 Free PMC article.
-
A Chimeric Japanese Encephalitis Vaccine Protects against Lethal Yellow Fever Virus Infection without Inducing Neutralizing Antibodies.mBio. 2020 Apr 7;11(2):e02494-19. doi: 10.1128/mBio.02494-19. mBio. 2020. PMID: 32265332 Free PMC article.
-
Identifying optimal capsid duplication length for the stability of reporter flaviviruses.Emerg Microbes Infect. 2020 Dec;9(1):2256-2265. doi: 10.1080/22221751.2020.1829994. Emerg Microbes Infect. 2020. PMID: 32981479 Free PMC article.
References
-
- Peltier DC, Lazear HM, Farmer JR, Diamond MS, Miller DJ. Neurotropic arboviruses induce interferon regulatory factor 3-mediated neuronal responses that are cytoprotective, interferon independent, and inhibited by Western equine encephalitis virus capsid. Journal of virology. 2013;87:1821–33. - PMC - PubMed
-
- Solomon T. Control of Japanese encephalitis-within our grasp? The New England journal of medicine. 2006;355:869–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

