Tyrosine Phosphorylation of an Actin-Binding Protein Girdin Specifically Marks Tuft Cells in Human and Mouse Gut
- PMID: 28375676
- PMCID: PMC5625852
- DOI: 10.1369/0022155417702586
Tyrosine Phosphorylation of an Actin-Binding Protein Girdin Specifically Marks Tuft Cells in Human and Mouse Gut
Abstract
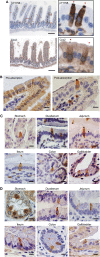
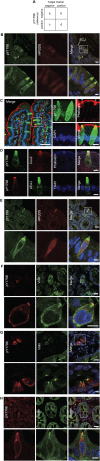
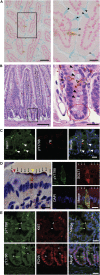
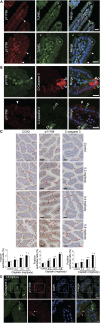
Tuft cells (TCs) are minor components of gastrointestinal epithelia, characterized by apical tufts and spool-shaped somas. The lack of reliable TC-markers has hindered the elucidation of its role. We developed site-specific and phosphorylation-status-specific antibodies against Girdin at tyrosine-1798 (pY1798) and found pY1798 immunostaining of mouse jejunum clearly depicted epithelial cells closely resembling TCs. This study aimed to validate pY1798 as a TC-marker. Double-fluorescence staining of intestines was performed with pY1798 and known TC-markers, for example, hematopoietic-prostaglandin-D-synthase (HPGDS), or doublecortin-like kinase 1 (DCLK1). Odds ratios (ORs) were calculated from cell counts to determine whether two markers were attracting (OR<1) or repelling (OR>1). In consequence, pY1798 signals strongly attracted those of known TC-markers. ORs for HPGDS in mouse stomach, small intestine, and colon were 0 for all, and 0.08 for DCLK1 in human small intestine. pY1798-positive cells in jejunum were distinct from other minor epithelial cells, including goblet, Paneth, and neuroendocrine cells. Thus, pY1798 was validated as a TC-marker. Interestingly, apoptosis inducers significantly increased relative TC frequencies despite the absence of proliferation at baseline. In conclusion, pY1798 is a novel TC-marker. Selective tyrosine phosphorylation and possible resistance to apoptosis inducers implied the activation of certain kinase(s) in TCs, which may become a clue to elucidate the enigmatic roles of TCs. .
Keywords: Girdin; brush cell; tuft cell marker; tyrosine phosphorylation.
Figures

Similar articles
-
Use of Anti-phospho-girdin Antibodies to Visualize Intestinal Tuft Cells in Free-Floating Mouse Jejunum Cryosections.J Vis Exp. 2018 Mar 21;(133):57475. doi: 10.3791/57475. J Vis Exp. 2018. PMID: 29630055 Free PMC article.
-
Girdin is phosphorylated on tyrosine 1798 when associated with structures required for migration.Biochem Biophys Res Commun. 2015 Mar 20;458(4):934-40. doi: 10.1016/j.bbrc.2015.02.065. Epub 2015 Feb 20. Biochem Biophys Res Commun. 2015. PMID: 25707853
-
Distribution of duodenal tuft cells is altered in pediatric patients with acute and chronic enteropathy.Biomed Res. 2020;41(2):113-118. doi: 10.2220/biomedres.41.113. Biomed Res. 2020. PMID: 32307399 Free PMC article.
-
Girdin, a novel actin-binding protein, and its family of proteins possess versatile functions in the Akt and Wnt signaling pathways.Ann N Y Acad Sci. 2006 Nov;1086:169-84. doi: 10.1196/annals.1377.016. Ann N Y Acad Sci. 2006. PMID: 17185515 Review.
-
An update on the biological characteristics and functions of tuft cells in the gut.Front Cell Dev Biol. 2023 Jan 10;10:1102978. doi: 10.3389/fcell.2022.1102978. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36704202 Free PMC article. Review.
Cited by
-
Novel protocol to observe the intestinal tuft cell using transmission electron microscopy.Biol Open. 2022 Feb 15;11(2):bio059007. doi: 10.1242/bio.059007. Epub 2022 Feb 16. Biol Open. 2022. PMID: 34994390 Free PMC article.
-
Luminal Chemosensory Cells in the Small Intestine.Nutrients. 2021 Oct 22;13(11):3712. doi: 10.3390/nu13113712. Nutrients. 2021. PMID: 34835968 Free PMC article. Review.
-
Single-Cell Transcriptomics Reveals a Conserved Metaplasia Program in Pancreatic Injury.Gastroenterology. 2022 Feb;162(2):604-620.e20. doi: 10.1053/j.gastro.2021.10.027. Epub 2021 Oct 23. Gastroenterology. 2022. PMID: 34695382 Free PMC article.
-
Regulation of immune responses by tuft cells.Nat Rev Immunol. 2019 Sep;19(9):584-593. doi: 10.1038/s41577-019-0176-x. Nat Rev Immunol. 2019. PMID: 31114038 Free PMC article. Review.
-
Colonic Tuft Cells: The Less-Recognized Therapeutic Targets in Inflammatory Bowel Disease and Colorectal Cancer.Int J Mol Sci. 2024 Jun 5;25(11):6209. doi: 10.3390/ijms25116209. Int J Mol Sci. 2024. PMID: 38892399 Free PMC article. Review.
References
-
- Jarvi O, Keyrilainen O. On the cellular structures of the epithelial invasions in the glandular stomach of mice caused by intramural application of 20-methylcholantren. Acta Pathol Microbiol Scand. 1956;39(Suppl 111):72–3. - PubMed
-
- Sato A. Tuft cells. Anat Sci Int. 2007;82:187–99. - PubMed
-
- Sato A, Hamano M, Miyoshi S. Increasing frequency of occurrence of tuft cells in the main excretory duct during postnatal development of the rat submandibular gland. Anat Rec. 1998;252:276–80. - PubMed
-
- Rhodin J, Dalhamn T. Electron microscopy of the tracheal ciliated mucosa in rat. Z Zellforsch Mikrosk Anat. 1956;44:345–412. - PubMed
-
- Hofer D, Asan E, Drenckhahn D. Chemosensory perception in the gut. News Physiol Sci. 1999;14: 18–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

